Ephrin-B2 Controls Pdgfrb Internalization and Signaling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Platelet-Derived Growth Factor Receptor Expression and Amplification in Choroid Plexus Carcinomas
Modern Pathology (2008) 21, 265–270 & 2008 USCAP, Inc All rights reserved 0893-3952/08 $30.00 www.modernpathology.org Platelet-derived growth factor receptor expression and amplification in choroid plexus carcinomas Nina N Nupponen1,*, Janna Paulsson2,*, Astrid Jeibmann3, Brigitte Wrede4, Minna Tanner5, Johannes EA Wolff 6, Werner Paulus3, Arne O¨ stman2 and Martin Hasselblatt3 1Molecular Cancer Biology Program, University of Helsinki, Helsinki, Finland; 2Department of Oncology–Pathology, Cancer Centrum Karolinska, Karolinska Institutet, Stockholm, Sweden; 3Institute of Neuropathology, University Hospital Mu¨nster, Mu¨nster, Germany; 4Department of Pediatric Oncology, University of Regensburg, Regensburg, Germany; 5Department of Oncology, Tampere University Hospital, Tampere, Finland and 6Children’s Cancer Hospital, MD Anderson Cancer Center, Houston, TX, USA Platelet-derived growth factor (PDGF) receptor signaling has been implicated in the development of glial tumors, but not yet been examined in choroid plexus carcinomas, pediatric tumors with dismal prognosis for which novel treatment options would be desirable. Therefore, protein expression of PDGF receptors a and b as well as amplification status of the respective genes, PDGFRA and PDGFRB, were examined in a series of 22 patients harboring choroid plexus carcinoma using immunohistochemistry and chromogenic in situ hybridization (CISH). The majority of choroid plexus carcinomas expressed PDGF receptors with 6 cases (27%) displaying high staining scores for PDGF receptor a and 13 cases (59%) showing high staining scores for PDGF receptor b. Correspondingly, copy-number gains of PDGFRA were observed in 8 cases out of 12 cases available for CISH and 1 case displayed amplification (six or more signals per nucleus). The proportion of choroid plexus carcinomas with amplification of PDGFRB was even higher (5/12 cases). -

Epha Receptors and Ephrin-A Ligands Are Upregulated by Monocytic
Mukai et al. BMC Cell Biology (2017) 18:28 DOI 10.1186/s12860-017-0144-x RESEARCHARTICLE Open Access EphA receptors and ephrin-A ligands are upregulated by monocytic differentiation/ maturation and promote cell adhesion and protrusion formation in HL60 monocytes Midori Mukai, Norihiko Suruga, Noritaka Saeki and Kazushige Ogawa* Abstract Background: Eph signaling is known to induce contrasting cell behaviors such as promoting and inhibiting cell adhesion/ spreading by altering F-actin organization and influencing integrin activities. We have previously demonstrated that EphA2 stimulation by ephrin-A1 promotes cell adhesion through interaction with integrins and integrin ligands in two monocyte/ macrophage cell lines. Although mature mononuclear leukocytes express several members of the EphA/ephrin-A subclass, their expression has not been examined in monocytes undergoing during differentiation and maturation. Results: Using RT-PCR, we have shown that EphA2, ephrin-A1, and ephrin-A2 expression was upregulated in murine bone marrow mononuclear cells during monocyte maturation. Moreover, EphA2 and EphA4 expression was induced, and ephrin-A4 expression was upregulated, in a human promyelocytic leukemia cell line, HL60, along with monocyte differentiation toward the classical CD14++CD16− monocyte subset. Using RT-PCR and flow cytometry, we have also shown that expression levels of αL, αM, αX, and β2 integrin subunits were upregulated in HL60 cells along with monocyte differentiation while those of α4, α5, α6, and β1 subunits were unchanged. Using a cell attachment stripe assay, we have shown that stimulation by EphA as well as ephrin-A, likely promoted adhesion to an integrin ligand- coated surface in HL60 monocytes. Moreover, EphA and ephrin-A stimulation likely promoted the formation of protrusions in HL60 monocytes. -
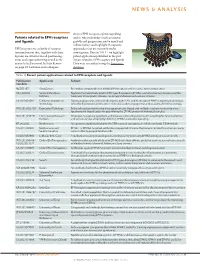
Patents Related to EPH Receptors and Ligands
NEWS & ANALYSIS discuss EPH receptor–ephrin signalling Patents related to EPH receptors and its role in disorders such as tumour and ligands growth and progression, nerve injury and inflammation, and highlight therapeutic EPH receptors are a family of receptor approaches that are currently under tyrosine kinases that, together with their investigation. Here in TABLE 1 we highlight ligands, are involved in cell positioning, patent applications published in the past tissue and organ patterning as well as the 3 years related to EPH receptors and ligands. control of cell survival. In their Review Data were researched using the Espacenet on page 39, Lackman and colleagues database. Table 1 | Recent patent applications related to EPH receptors and ligands Nature Reviews | Drug Discovery Publication Applicants Subject numbers NZ 581397 AstraZeneca Pyrimidine compounds that inhibit EPH receptors and are useful for treating cancer HK 1108702 Sanford-Burnham Peptides that selectively bind to EPH type-B receptors (EPHBs); useful for tumour imaging and the Institute treatment of neoplastic disease, neurological disease and vascular disease US 2013091591 California Institute of During angiogenesis, arterial cells express ephrin B2, and its receptor EPHB4 is expressed on venous Technology cells; this distinction can be used in methods to alter angiogenesis and to assess the effect of drugs WO 2013052710 Expression Pathology Selected reaction monitoring mass spectrometry-based and multiple reaction monitoring mass spectrometry-based assays for quantifying -

Trastuzumab and Pertuzumab in Circulating Tumor DNA ERBB2-Amplified HER2-Positive Refractory Cholangiocarcinoma
www.nature.com/npjprecisiononcology CASE REPORT OPEN Trastuzumab and pertuzumab in circulating tumor DNA ERBB2-amplified HER2-positive refractory cholangiocarcinoma Bhavya Yarlagadda 1, Vaishnavi Kamatham1, Ashton Ritter1, Faisal Shahjehan1 and Pashtoon M. Kasi2 Cholangiocarcinoma is a heterogeneous and target-rich disease with differences in actionable targets. Intrahepatic and extrahepatic types of cholangiocarcinoma differ significantly in clinical presentation and underlying genetic aberrations. Research has shown that extrahepatic cholangiocarcinoma is more likely to be associated with ERBB2 (HER2) genetic aberrations. Various anti-HER2 clinical trials, case reports and other molecular studies show that HER2 is a real target in cholangiocarcinoma; however, anti-HER2 agents are still not approved for routine administration. Here, we show in a metastatic cholangiocarcinoma with ERBB2 amplification identified on liquid biopsy (circulating tumor DNA (ctDNA) testing), a dramatic response to now over 12 months of dual-anti-HER2 therapy. We also summarize the current literature on anti-HER2 therapy for cholangiocarcinoma. This would likely become another treatment option for this target-rich disease. npj Precision Oncology (2019) 3:19 ; https://doi.org/10.1038/s41698-019-0091-4 INTRODUCTION We present a 71-year-old female diagnosed with metastatic Cholangiocarcinoma (CCA) is a lethal tumor arising from the CCA with ERBB2 (HER2) 3+ amplification determined by circulating epithelium of the bile ducts that most often presents at an tumor DNA (ctDNA) -

DNA Damage and the Activation of the P53 Pathway Mediate Alterations in Metabolic and Secretory Functions of Adipocytes
3062 Diabetes Volume 65, October 2016 Bastien Vergoni,1,2 Pierre-Jean Cornejo,1,2 Jérôme Gilleron,1,2 Mansour Djedaini,1,2 Franck Ceppo,1,2 Arnaud Jacquel,2,3 Gwennaelle Bouget,1,2 Clémence Ginet,1,2 Teresa Gonzalez,1,2,4 Julie Maillet,5,6,7 Véronique Dhennin,5,6,7 Marie Verbanck,5,6,7 Patrick Auberger,2,3 Philippe Froguel,5,6,7,8 Jean-François Tanti,1,2 and Mireille Cormont1,2 DNA Damage and the Activation of the p53 Pathway Mediate Alterations in Metabolic and Secretory Functions of Adipocytes Diabetes 2016;65:3062–3074 | DOI: 10.2337/db16-0014 Activation of the p53 pathway in adipose tissue contributes Insulin resistance is associated with obesity and is a major to insulin resistance associated with obesity. However, the risk factor for type 2 diabetes. Adipose tissue (AT) plays mechanisms of p53 activation and the effect on adipocyte an important role in glucose and lipid homeostasis (1,2), functions are still elusive. Here we found a higher level of and AT dysfunction associated with obesity has emerged DNA oxidation and a reduction in telomere length in adipose as a critical event for the development of insulin resis- tissueofmicefedahigh-fatdietandanincreaseinDNA tance. Low-grade inflammation and oxidative and hypoxic damage and activation of the p53 pathway in adipocytes. stresses both develop in obese AT and are involved in the Interestingly, hallmarks of chronic DNA damage are visible alteration of adipocyte functions (3–6). Therefore, activa- at the onset of obesity. Furthermore, injection of lean mice tion of stress-signaling pathways in adipocytes is critically with doxorubicin, a DNA damage-inducing drug, increased involved in AT dysfunction and insulin resistance (7–10). -

Ephb3 Suppresses Non-Small-Cell Lung Cancer Metastasis Via a PP2A/RACK1/Akt Signalling Complex
ARTICLE Received 7 Nov 2011 | Accepted 11 Jan 2012 | Published 7 Feb 2012 DOI: 10.1038/ncomms1675 EphB3 suppresses non-small-cell lung cancer metastasis via a PP2A/RACK1/Akt signalling complex Guo Li1, Xiao-Dan Ji1, Hong Gao1, Jiang-Sha Zhao1, Jun-Feng Xu1, Zhi-Jian Sun1, Yue-Zhen Deng1, Shuo Shi1, Yu-Xiong Feng1, Yin-Qiu Zhu1, Tao Wang2, Jing-Jing Li1 & Dong Xie1 Eph receptors are implicated in regulating the malignant progression of cancer. Here we find that despite overexpression of EphB3 in human non-small-cell lung cancer, as reported previously, the expression of its cognate ligands, either ephrin-B1 or ephrin-B2, is significantly downregulated, leading to reduced tyrosine phosphorylation of EphB3. Forced activation of EphB3 kinase in EphB3-overexpressing non-small-cell lung cancer cells inhibits cell migratory capability in vitro as well as metastatic seeding in vivo. Furthermore, we identify a novel EphB3-binding protein, the receptor for activated C-kinase 1, which mediates the assembly of a ternary signal complex comprising protein phosphatase 2A, Akt and itself in response to EphB3 activation, leading to reduced Akt phosphorylation and subsequent inhibition of cell migration. Our study reveals a novel tumour-suppressive signalling pathway associated with kinase-activated EphB3 in non-small-cell lung cancer, and provides a potential therapeutic strategy by activating EphB3 signalling, thus inhibiting tumour metastasis. 1 Key Laboratory of Nutrition and Metabolism, Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences and Graduate School of Chinese Academy of Sciences, Shanghai 200031, China. 2 The Eastern Hepatobiliary Surgery Hospital, the Second Military Medical University, Shanghai 200433, China. -

Engrailed and Fgf8 Act Synergistically to Maintain the Boundary Between Diencephalon and Mesencephalon Scholpp, S., Lohs, C
5293 Erratum Engrailed and Fgf8 act synergistically to maintain the boundary between diencephalon and mesencephalon Scholpp, S., Lohs, C. and Brand, M. Development 130, 4881-4893. An error in this article was not corrected before going to press. Throughout the paper, efna4 should be read as EphA4, as the authors are referring to the gene encoding the ephrin A4 receptor (EphA4) and not to that encoding its ligand ephrin A4. We apologise to the authors and readers for this mistake. Research article 4881 Engrailed and Fgf8 act synergistically to maintain the boundary between diencephalon and mesencephalon Steffen Scholpp, Claudia Lohs and Michael Brand* Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG), Dresden, Germany Department of Genetics, University of Dresden (TU), Pfotenhauer Strasse 108, 01307 Dresden, Germany *Author for correspondence (e-mail: [email protected]) Accepted 23 June 2003 Development 130, 4881-4893 © 2003 The Company of Biologists Ltd doi:10.1242/dev.00683 Summary Specification of the forebrain, midbrain and hindbrain However, a patch of midbrain tissue remains between the primordia occurs during gastrulation in response to signals forebrain and the hindbrain primordia in such embryos. that pattern the gastrula embryo. Following establishment This suggests that an additional factor maintains midbrain of the primordia, each brain part is thought to develop cell fate. We find that Fgf8 is a candidate for this signal, as largely independently from the others under the influence it is both necessary and sufficient to repress pax6.1 and of local organizing centers like the midbrain-hindbrain hence to shift the DMB anteriorly independently of the boundary (MHB, or isthmic) organizer. -

Selective Insulin Signaling Through a and B Insulin Receptors Regulates Transcription of Insulin and Glucokinase Genes in Pancreatic  Cells
Molecular Cell, Vol. 7, 559±570, March, 2001, Copyright 2001 by Cell Press Selective Insulin Signaling through A and B Insulin Receptors Regulates Transcription of Insulin and Glucokinase Genes in Pancreatic  Cells Barbara Leibiger,*§ Ingo B. Leibiger,*§k ceptors as the primary target, include signaling via mito- Tilo Moede,* Sabine Kemper,* gen-activated protein (MAP) kinases and phosphoinosi- Rohit N. Kulkarni,² C. Ronald Kahn,² tol-3 kinase (PI3K). The insulin receptor (IR), the first Lina Moitoso de Vargas,³ and Per-Olof Berggren* step in these cascades, exists in two isoforms as a result *The Rolf Luft Center for Diabetes Research of alternative mRNA splicing of the 11th exon of the insulin Department of Molecular Medicine proreceptor transcript (Seino et al., 1989). The A type Karolinska Institutet (IR-A), or Ex11Ϫ (Ullrich et al., 1985), lacks whereas the S-171 76 Stockholm B type (IR-B), or Ex11ϩ (Ebina et al., 1985), contains Sweden the respective sequence coding for 12 amino acids in ² Research Division the C terminus of the ␣ chain of the receptor. To date, Joslin Diabetes Center and no insulin-induced effect has been reported that dis- Department of Medicine criminates signaling via A- and B-type receptors. In fact, Harvard Medical School the functional significance of these IR isoforms remains Boston, Massachusetts 02215 unclear. ³ Department of Medicine Recent studies have shown that the insulin-producing New England Medical Center and pancreatic  cell is a target for insulin action, with insulin Tufts University School of Medicine effects on transcription, translation, Ca2ϩ flux, and exo- Boston, Massachusetts 02111 cytosis (Leibiger et al., 1998a, 2000; Xu and Rothenberg, 1998; Xu et al., 1998; Aspinwall et al., 1999; Kulkarni et al., 1999a). -

On the Turning of Xenopus Retinal Axons Induced by Ephrin-A5
Development 130, 1635-1643 1635 © 2003 The Company of Biologists Ltd doi:10.1242/dev.00386 On the turning of Xenopus retinal axons induced by ephrin-A5 Christine Weinl1, Uwe Drescher2,*, Susanne Lang1, Friedrich Bonhoeffer1 and Jürgen Löschinger1 1Max-Planck-Institute for Developmental Biology, Spemannstrasse 35, 72076 Tübingen, Germany 2MRC Centre for Developmental Neurobiology, King’s College London, New Hunt’s House, Guy’s Hospital Campus, London SE1 1UL, UK *Author for correspondence (e-mail: [email protected]) Accepted 13 January 2003 SUMMARY The Eph family of receptor tyrosine kinases and their turning or growth cone collapse when confronted with ligands, the ephrins, play important roles during ephrin-A5-Fc bound to beads. However, when added in development of the nervous system. Frequently they exert soluble form to the medium, ephrin-A5 induces growth their functions through a repellent mechanism, so that, for cone collapse, comparable to data from chick. example, an axon expressing an Eph receptor does not The analysis of growth cone behaviour in a gradient of invade a territory in which an ephrin is expressed. Eph soluble ephrin-A5 in the ‘turning assay’ revealed a receptor activation requires membrane-associated ligands. substratum-dependent reaction of Xenopus retinal axons. This feature discriminates ephrins from other molecules On fibronectin, we observed a repulsive response, with the sculpturing the nervous system such as netrins, slits and turning of growth cones away from higher concentrations class 3 semaphorins, which are secreted molecules. While of ephrin-A5. On laminin, retinal axons turned towards the ability of secreted molecules to guide axons, i.e. -

Pdgfrβ Regulates Adipose Tissue Expansion and Glucose
1008 Diabetes Volume 66, April 2017 Yasuhiro Onogi,1 Tsutomu Wada,1 Chie Kamiya,1 Kento Inata,1 Takatoshi Matsuzawa,1 Yuka Inaba,2,3 Kumi Kimura,2 Hiroshi Inoue,2,3 Seiji Yamamoto,4 Yoko Ishii,4 Daisuke Koya,5 Hiroshi Tsuneki,1 Masakiyo Sasahara,4 and Toshiyasu Sasaoka1 PDGFRb Regulates Adipose Tissue Expansion and Glucose Metabolism via Vascular Remodeling in Diet-Induced Obesity Diabetes 2017;66:1008–1021 | DOI: 10.2337/db16-0881 Platelet-derived growth factor (PDGF) is a key factor in The physiological roles of the vasculature in adipose tissue angiogenesis; however, its role in adult obesity remains have been attracting interest from the viewpoint of adipose unclear. In order to clarify its pathophysiological role, tissue expansion and chronic inflammation (1,2). White we investigated the significance of PDGF receptor b adipose tissue (WAT) such as visceral fat possesses the (PDGFRb) in adipose tissue expansion and glucose unique characteristic of plasticity; its volume may change metabolism. Mature vessels in the epididymal white several fold even after growth depending on nutritional adipose tissue (eWAT) were tightly wrapped with peri- conditions. Enlarged adipose tissue is chronically exposed cytes in normal mice. Pericyte desorption from vessels to hypoxia (3,4), which stimulates the production of angio- and the subsequent proliferation of endothelial cells genic factors for the supplementation of nutrients and were markedly increased in the eWAT of diet-induced oxygen to the newly enlarged tissue area (5). Selective ab- obese mice. Analyses with flow cytometry and adipose lation of the vasculature in WAT by apoptosis-inducible tissue cultures indicated that PDGF-B caused the de- peptides or the systemic administration of angiogenic in- PATHOPHYSIOLOGY tachment of pericytes from vessels in a concentration- hibitors has been shown to reduce WAT volumes and result dependent manner. -

Insulin–Insr Signaling Drives Multipotent Progenitor Differentiation Toward Lymphoid Lineages
Article Insulin–InsR signaling drives multipotent progenitor differentiation toward lymphoid lineages Pengyan Xia,1* Shuo Wang,1* Ying Du,1 Guanling Huang,1,2 Takashi Satoh,3 Shizuo Akira,3 and Zusen Fan1 1Key Laboratory of Infection and Immunity of CAS, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China 2University of Chinese Academy of Sciences, Beijing 100049, China 3Department of Host Defense, Research Institute for Microbial Diseases (RIMD), Osaka University, Suita, Osaka 565-0871, Japan The lineage commitment of HSCs generates balanced myeloid and lymphoid populations in hematopoiesis. However, the un- derlying mechanisms that control this process remain largely unknown. Here, we show that insulin–insulin receptor (InsR) signaling is required for lineage commitment of multipotent progenitors (MPPs). Deletion of Insr in murine bone marrow causes skewed differentiation of MPPs to myeloid cells. mTOR acts as a downstream effector that modulates MPP differentiation. mTOR activates Stat3 by phosphorylation at serine 727 under insulin stimulation, which binds to the promoter of Ikaros, lead- ing to its transcription priming. Our findings reveal that the insulin–InsR signaling drives MPP differentiation into lymphoid lineages in early lymphopoiesis, which is essential for maintaining a balanced immune system for an individual organism. Hematopoiesis is the process of producing all compo- Insulin, as the primary anabolic hormone, modulates a nents of the blood system from hematopoietic stem cells variety of physiological processes, including growth, differ- (HSCs; Naik et al., 2013; Mendelson and Frenette, 2014; entiation, apoptosis, and synthesis and breakdown of lipid, Walter et al., 2015). HSCs are quiescent, self-renewable pro- protein, and glucose (Samuel and Shulman, 2012). -

60+ Genes Tested FDA-Approved Targeted Therapies & Gene Indicators
® 60+ genes tested ABL1, ABL2, ALK, AR, ARAF, ATM, ATR, BRAF, BRCA1*, BRCA2*, BTK, CCND1, CCND2, CCND3, CDK4, Somatic mutation detection CDK6, CDKN1A, CDKN1B, CDKN2A, CDKN2B, DDR1, DDR2, EGFR, ERBB2 (HER2), ESR1, FGFR1, FGFR2, for approved cancer therapies FGFR3, FGFR4, FLCN, FLT1, FLT3, FLT4, GNA11, GNAQ, HDAC1, HDAC2, HRAS, JAK1, JAK2, KDR, KIT, in solid tumors KRAS, MAP2K1, MET, MTOR, NF1, NF2, NRAS, PALB2, PARP1, PDGFRA, PDGFRB, PIK3CA, PIK3CD, PTCH1, PTEN, RAF1, RET, ROS1, SMO, SRC, STK11, TNK2, TSC1, TSC2 FDA-approved Targeted Therapies & Gene Indicators Abiraterone AR Necitumumab EGFR Ado-Trastuzumab ERBB2 (HER2) Nilotinib ABL1, ABL2, DDR1, DDR2, KIT, PDGFRA, PDGFRB Emtansine FGFR1, FGFR2, FGFR3, FLT1, FLT4, KDR, PDGFRA, Afatinib EGFR, ERBB2 (HER2) Nintedanib PDGFRB Alectinib ALK Olaparib ATM, ATR, BRCA1*, BRCA2*, PALB2, PARP1 Anastrozole ESR1 Osimertinib EGFR Axitinib FLT1, FLT4, KDR, KIT, PDGFRA, PDGFRB CDK4, CDK6, CCND1, CCND2, CCND3, CDKN1A, Palbociclib Belinostat HDAC1, HDAC2 CDKN1B, CDKN2A, CDKN2B Panitumumab EGFR Bicalutamide AR Panobinostat HDAC1, HDAC2 Bosutinib ABL1, SRC Pazopanib FLT1, FLT4, KDR, KIT, PDGFRA, PDGFRB Cabozantinib FLT1, FLT3, FLT4, KDR, KIT, MET, RET Pertuzumab ERBB2 (HER2) Ceritinib ALK ABL1, FGFR1, FGFR2, FGFR3, FGFR4, FLT1, FLT3, FLT4, Cetuximab EGFR Ponatinib KDR, KIT, PDGFRA, PDGFRB, RET, SRC Cobimetinib MAP2K1 Ramucirumab KDR Crizotinib ALK, MET, ROS1 Regorafenib ARAF, BRAF, FLT1, FLT4, KDR, KIT, PDGFRB, RAF1, RET Dabrafenib BRAF Ruxolitinib JAK1, JAK2 Dasatinib ABL1, ABL2, DDR1, DDR2, SRC, TNK2