Development of Palladium-Catalyzed Decarboxylative Benzylation Reactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A-PDF Split DEMO : Purchase from to Remove the Watermark
A-PDF Split DEMO : Purchase from www.A-PDF.com to remove the watermark Figure 7.5 (a) Transition state for E2H-syn elimination. (b) Transition state for E2H-anti elimination. (c) Transition state for E,C elimination. tion state similar to that shown in Figure 7.5~is expected, since base attacks the molecule backside to the leaving group but frontside to the /3 hydrogen. Let us now turn to the experimental results to see if these predictions are borne out in fact. It has long been known that E2H reactions normally give preferentially anti elimination. For example, reaction of meso-stilbene dibromide with potassium ethoxide gives cis-bromostilbene (Reaction 7.39), whereas reaction of the D,L-dibromide gives the trans product (Reaction 7.40).lo2 A multitude of other examples exist-see, for example, note 64 (p. 355) and note 82 (p. 362). E2H reactions do, however, give syn elimination when: (1) an H-X di- hedral angle of 0" is achievable but one of 180" is not or, put another way, H and X can become syn-periplanar but not trans-periplanar ; (2) a syn hydrogen is much more reactive than the anti ones; (3) syn elimination is favored for steric reasons; and (4) an anionic base that remains coordinated with its cation, that, in turn, is coordinated with the leaving group, is used as catalyst. The very great importance of category 4 has only begun to be fully realized in the early 1970s. lo2 P. Pfeiffer, 2. Physik. Chem. Leibrig, 48, 40 (1904). 1,2-Elimination Reactions 371 An example of category 1 is found in the observation by Brown and Liu that eliminations from the rigid ring system 44, induced by the sodium salt of 2- cyclohexylcyclohexanol in triglyme, produces norborene (98 percent) but no 2-de~teronorbornene.~~~The dihedral angle between D and tosylate is O", but Crown ether present: No Yes that between H and tosylate is 120". -
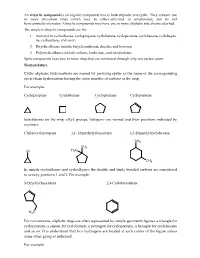
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis
New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Author: Jason Joseph Marineau Persistent link: http://hdl.handle.net/2345/1741 This work is posted on eScholarship@BC, Boston College University Libraries. Boston College Electronic Thesis or Dissertation, 2010 Copyright is held by the author, with all rights reserved, unless otherwise noted. Boston College The Graduate School of Arts and Sciences Department of Chemistry NEW APPLICATIONS OF CYCLOBUTADIENE CYCLOADDITIONS: DIVERSITY AND TARGET ORIENTED SYNTHESIS a dissertation by JASON JOSEPH MARINEAU submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2010 © Copyright by JASON JOSEPH MARINEAU 2010 New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Jason J. Marineau Thesis Advisor: Professor Marc. L. Snapper Abstract Cyclobutadiene cycloadditions provide rapid access to rigid polycyclic systems with high strain energy and unusual molecular geometries. Further functionalization of these systems allows entry into unexplored chemical space. A tricarbonylcyclobutadiene iron complex on solid support enables exploration of these cycloadditions in a parallel format amenable to diversity oriented synthesis. Modeling of the cycloaddition transition states with density functional calculations provides a theoretical basis for analysis of the regioselectivity observed in generation of these substituted bicyclo[2.2.0]hexene derivatives. The high strain energy accessible in cyclobutadiene cycloadducts and their derivatives renders them useful synthons for access to medium-ring natural products through ring expansion. Torilin, a guaiane sesquiterpene isolated from extracts of the fruits of Torilis japonica, exhibits a range of biological activities including testosterone 5 -reductase inhibition, hKv1.5 channel blocking, hepatoprotective, anti-inflammatory and anti-cancer effects. -

Palladium-Catalyzed Tsuji-Trost Allylation of J-Borylated Allyl Acetates
ANNÉE 2013 THÈSE / UNIVERSITÉ DE RENNES 1 sous le sceau de l’Université Européenne de Bretagne pour le grade de DOCTEUR DE L’UNIVERSITÉ DE RENNES 1 Mention : CHIMIE Ecole doctorale Sciences de la Matière de Rennes présentée par Krishna Kishore Kukkadapu UMR 6510 CNRS Chimie et Photonique Moléculaires UFR Sciences et Propriétés de la Matière Thèse soutenue à Rennes Gamma-borylated le Jeudi 6 juin 2013 allylic acetates as 3 devant le jury composé de : Véronique BELLOSTA carbon functionalized Professeur –ESPCI / rapporteur units : synthesis and Stéphane PELLET-ROSTAING Chargé de recherche CNRS à l’ICSM-CEA / applications rapporteur Florence MONGIN Professeur à l’Université de Renne1 / / examinateur Mathieu PUCHEAULT Chargé de recherche CNRS / examinateur Michel VAULTIER Directeur de recherche CNRS/ / directeur de thèse 1 Table of contents : Résumé de la thèse en français 5 Acknowledgements: 22 Abbreviations: 24 General Introduction: 27 PART ͲA 30 Chapter I: Bibliography 30 I. 1. Synthesis &applications of JͲborylated allylic electrophiles: 31 I. 1. i. Synthesis of JͲborylated allylic electrophiles: 31 I. 1. ii. Applications of JͲborylated allylic electrophiles: 33 I. 1. ii. a. In iridium catalysis: 33 I. 1. ii. b. In copper catalysis: 37 I. 1. ii. c. In palladium catalysis: 39 I. 1. ii. d. In Grignard reaction: 41 I. 1. ii. e. In Diels Alder reaction: 42 I. 1. ii. f. In Mitsunobu reaction: 43 I. 1. ii. g. In cyclopropane synthesis: 46 I. 2. Tsuji ͲTrost Allylation: 48 I. 2. i. Stereochemistry in Tsuji ͲTrost allylation: 51 I. 2. ii. Regioselectivity in Tsuji ͲTrost allylation: 52 I. 2. iii. -

Here Science-Based Cost- Effective Pathways Forward?
Sponsors Institute of Atomic and Molecular Sciences, Academia Sinica, Taiwan PIRE-ECCI Program, UC Santa Barbara, USA Max-Planck-Gesellschaft, Germany National Science Council, Taiwan Organizing committee Dr. Kuei-Hsien Chen (IAMS, Academia Sinica, Taiwan) Dr. Susannah Scott (University of California - Santa Barbara, USA) Dr. Alec Wodtke (University of Göttingen & Max-Planck-Gesellschaft, Germany) Table of Contents General Information .............................................................................................. 1 Program for Sustainable Energy Workshop ....................................................... 3 I01 Dr. Alec M. Wodtke Beam-surface Scattering as a Probe of Chemical Reaction Dynamics at Interfaces ........................................................................................................... 7 I02 Dr. Kopin Liu Imaging the steric effects in polyatomic reactions ............................................. 9 I03 Dr. Chi-Kung Ni Energy Transfer of Highly Vibrationally Excited Molecules and Supercollisions ................................................................................................. 11 I04 Dr. Eckart Hasselbrink Energy Conversion from Catalytic Reactions to Hot Electrons in Thin Metal Heterostructures ............................................................................................... 13 I05 Dr. Jim Jr-Min Lin ClOOCl and Ozone Hole — A Catalytic Cycle that We Don’t Like ............... 15 I06 Dr. Trevor W. Hayton Nitric Oxide Reduction Mediated by a Nickel Complex -

October 1, 2020 Webinar
Phosphorus Specialties: the cornerstone of synthesis for pharmaceutical applications Eamonn Conrad, Ph.D., Global BD Manager Dino Amoroso, Ph.D., NA Account Manager William Stibbs, Ph.D. Senior BD Manager Overview • Solvay Phosphorus Specialties, Strem Chemicals Inc. Partnership • Chemistry for the Manufacture of Phosphine Ligands • Applications in Pharmaceutical Catalysis • Applications in Life Sciences • Summary and Questions *Solvay partners with Strem Chemicals for sample distribution page 2 Phosphorus Specialties Strem Chemicals, Inc. Solvay partners with Strem Chemicals for sample distribution! Established in 1964 More than 55 years of experience in manufacturing and handling high quality inorganics and organometallics 5,000+ specialty chemicals available Laboratory Chemicals for R&D cGMP Products Manufactured in Kilo-lab Suites High Pressure Materials Custom Synthesis Projects Customers include: Corporate Headquarters Academic, industrial and government R&D laboratories Corporate Headquarters European Headquarters Commercial scale businesses in the pharmaceutical, Newburyport, MA USA Strasbourg, France microelectronics, chemical & petrochemical industries Phosphorus Specialties Samples available from Strem Who We Are Phosphorus Specialties Mining Solutions Polymer Additives Putting our science to work for customers to develop differentiated products and technologies Dedicated on-site technical service and applications expertise to support our customers’ needs Deep customer relationships and ongoing collaborations to solve demanding -

Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to Α
PHOSPHINE-CATALYZED ADDITIONS OF NUCLEOPHILES AND ELECTROPHILES TO α,β–UNSATURATED CARBONYL COMPOUNDS Reported by Michael Scott Bultman November 4, 2004 INTRODUCTION Organophosphorous compounds are becoming increasingly important in organic synthesis. Phosphines serve as precursors to phosphonium ylides in the Wittig reaction,1 and as nucleophilic triggers in the Mitsunobu2 and Staudinger3 reactions. In these processes, the phosphine is stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained attention in the last ten years. First reported by Rauhut and Currier in 1963,5 phosphine catalysis has since been reinvestigated after the phosphine ligands in some transition-metal-catalyzed reactions were found to be better catalysts than the metal/phosphine complexes alone!6 Phosphines are well suited for catalyzing the addition of both nucleophiles and electrophiles to electron deficient alkenes, alkynes, and allenes. Activation of these α,β-unsaturated carbonyl systems with the phosphine enables the formation of new bonds at the α-, β-, and γ-positions. This report will highlight these different modes of addition to α,β-unsaturated carbonyl systems under phosphine catalysis that allow for the formation of a wide array of products from a single class of substrates. GENERAL REACTIVITY OF PHOSPHINES Key characteristics required for successful nucleophilic catalysis lie in the balance of leaving group ability, nucleophilicity, and ease of ylid formation. Increasing leaving group ability can often be + correlated with decreasing basicity. -

Guidelines for Pyrophoric Materials
Guidelines for Pyrophoric Materials Definition and Hazards Pyrophoric materials are substances that ignite instantly upon exposure to air, moisture in the air, oxygen or water. Other common hazards include corrosivity, teratogenicity, and organic peroxide formation, along with damage to the liver, kidneys, and central nervous system. Examples include metal hydrides, finely divided metal powders, nonmetal hydride and alkyl compounds, white phosphorus, alloys of reactive materials and organometallic compounds, including alkylithiums. Additional pyrophoric materials are listed in Appendix A. Failure to follow proper handling techniques could result in serious injury or death. Controlling the Hazards . If possible, use safer chemical alternatives. A “dry run” of the experiment should be performed using low-hazard materials, such as water or solvent, as appropriate. Limit the amount purchased and the amount stored. Do not accumulate unneeded pyrophoric materials. BEFORE working with pyrophoric materials, read the MSDS sheets. The MSDS must be reviewed before using an unfamiliar chemical and periodically as a reminder. A Standard Operating Procedure (SOP) should be prepared and reviewed for each process involving pyrophoric materials. In lab training should be completed and documented. If possible, use the “buddy system”. Working alone with pyrophorics is strongly discouraged. All glassware used for pyrophorics should be oven-dried and free of moisture. Review the location of the safety shower, eyewash, telephone, and fire extinguisher. Keep an appropriate fire extinguisher or extinguishing material close at hand. Additional controls when handling liquid pyrophoric materials . Secure pyrophoric reagent bottle to stand. Secure the syringe so if the plunger blows out of the body of the syringe the contents will not splash anyone. -

The X1 Method for Accurate and Efficient Prediction of Heats of Formation
THE JOURNAL OF CHEMICAL PHYSICS 127, 214105 ͑2007͒ The X1 method for accurate and efficient prediction of heats of formation ͒ Jianming Wu and Xin Xua State Key Laboratory of Physical Chemistry of Solid Surfaces and Center for Theoretical Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China ͑Received 31 July 2007; accepted 25 September 2007; published online 5 December 2007͒ We propose the X1 method which combines the density functional theory method with a neural network ͑NN͒ correction for an accurate yet efficient prediction of heats of formation. It calculates the final energy by using B3LYP/6-311+G͑3df,2p͒ at the B3LYP/6-311+G͑d,p͒ optimized geometry to obtain the B3LYP standard heats of formation at 298 K with the unscaled zero-point energy and thermal corrections at the latter basis set. The NN parameters cover 15 elements of H, Li, Be, B, C, N, O, F, Na, Mg, Al, Si, P, S, and Cl. The performance of X1 is close to the Gn theories, giving a mean absolute deviation of 1.43 kcal/mol for the G3/99 set of 223 molecules up to 10 nonhydrogen atoms and 1.48 kcal/mol for the X1/07 set of 393 molecules up to 32 nonhydrogen atoms. © 2007 American Institute of Physics. ͓DOI: 10.1063/1.2800018͔ I. INTRODUCTION the B3LYP method to calculate heats of formation of neutral organic molecules. Their results are promising. Calibrated ͑⌬ ͒ The accurate prediction of heats of formation Hf is with their own compilation of 180 organic molecules for the one of the central topics in computational chemistry. -

Bond Formation Reactions to Phosphorus Using an Electrophilic Phosphinidene Complex
BOND FORMATION REACTIONS TO PHOSPHORUS USING AN ELECTROPHILIC PHOSPHINIDENE COMPLEX A Thesis Submitted to the Faculty of Graduate Studies and Research In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy in Chemistry University of Regina By Kandasamy Vaheesar Regina, Saskatchewan September, 2013 Copyright 2013: K. Vaheesar UNIVERSITY OF REGINA FACULTY OF GRADUATE STUDIES AND RESEARCH SUPERVISORY AND EXAMINING COMMITTEE Kandasamy Vaheesar, candidate for the degree of Doctor of Philosophy in Chemistry, has presented a thesis titled, Bond Formation Reactions to Phosphorus Using an Electrophilic Phosphinidene Complex, in an oral examination held on August 28, 2013. The following committee members have found the thesis acceptable in form and content, and that the candidate demonstrated satisfactory knowledge of the subject material. External Examiner: *Dr. Stephen Foley, University of Saskatchewan Supervisor: Dr. Brian Sterenberg, Department of Chemistry/Biochemistry Committee Member: Dr. Mauricio Barbi, Department of Physics Committee Member: Dr. Allan East, Department of Chemistry/Biochemistry Committee Member: Dr. R. Scott Murphy, Department of Chemistry/Biochemistry Chair of Defense: Dr. Dongyan Blachford, Faculty of Graduate Studies & Research *Participated via Video Conference ABSTRACT Electrophilic phosphinidene complexes play a central role in organophosphorus chemistry. The chemistry of transient phosphinidene complexes has been well studied, but stable, cationic phosphinidene complexes are not as well understood. Therefore the i + 5 reactivity of a cationic phosphinidene complex [CpFe(CO)2{PN Pr2}] (Cp = η - cyclopentadienyl, iPr = isopropyl), toward bond activation, cycloaddition and nucleophilic addition has been examined. i + The complex [CpFe(CO)2{PN Pr2}] reacts with primary, secondary, and tertiary i + silanes to form the silyl phosphine complexes [CpFe(CO)2{P(H)(SiR3)N Pr2}] (SiR3 = SiPhH2, SiPh2H, Si(C2H5)3), in which the phosphinidene has inserted into the Si-H bond. -

Keto Esters Vincent Bizet, Valérie Lefebvre, Jérôme Baudoux, Marie-Claire Lasne, Agathe Boulangé, Stéphane Leleu, Xavier Franck, Jacques Rouden
Metal-Free SN2’ Decarboxylative Rearrangement of β-Keto Esters Vincent Bizet, Valérie Lefebvre, Jérôme Baudoux, Marie-Claire Lasne, Agathe Boulangé, Stéphane Leleu, Xavier Franck, Jacques Rouden To cite this version: Vincent Bizet, Valérie Lefebvre, Jérôme Baudoux, Marie-Claire Lasne, Agathe Boulangé, et al.. Metal- Free SN2’ Decarboxylative Rearrangement of β-Keto Esters. European Journal of Organic Chemistry, Wiley-VCH Verlag, 2011, 2011 (22), pp.4170-4175. 10.1002/ejoc.201100120. hal-01017341 HAL Id: hal-01017341 https://hal.archives-ouvertes.fr/hal-01017341 Submitted on 29 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. FULL PAPER DOI: 10.1002/ejoc.201100120 Ј Metal-Free SN2 Decarboxylative Rearrangement of β-Keto Esters Vincent Bizet,[a] Valérie Lefebvre,[a] Jérôme Baudoux,*[a] Marie-Claire Lasne,[a] Agathe Boulangé,[b] Stéphane Leleu,*[b] Xavier Franck,[b] and Jacques Rouden*[a] Keywords: Nucleophilic substitution / Reaction mechanisms / Organocatalysis / Rearrangement/ Decarboxylation Treatment of 2-methoxycarbonyl- (or 3-cyano-)allyl aceto- tested to elucidate the mechanism. The results of the study acetates with a tertiary amine or triphenylphosphane af- strongly suggest an intermolecular pathway involving a forded α-methylene γ-substituted δ-keto esters (or nitrile) in SN2Ј–decarboxylation–SN2Ј cascade. -

Stretchable Chiral Pockets for Palladium-Catalyzed Highly Chemo- and Enantioselective Allenylation ✉ ✉ Yuchen Zhang1, Xue Zhang2 & Shengming Ma 1,2
ARTICLE https://doi.org/10.1038/s41467-021-22498-1 OPEN Stretchable chiral pockets for palladium-catalyzed highly chemo- and enantioselective allenylation ✉ ✉ Yuchen Zhang1, Xue Zhang2 & Shengming Ma 1,2 Pyrazolones are a vital class of heterocycles possessing various biological properties and much attention is paid to the diversified synthesis of enantiopure pyrazolone derivatives. We describe here the development of diphenylphosphinoalkanoic acid based chiral bisphosphine 1234567890():,; ligands, which are successfully applied to the palladium-catalyzed asymmetric allenylation of racemic pyrazol-5-ones. The reaction affords C-allenylation products, optically active pyrazol- 5-ones bearing an allene unit, in high chemo- and enantioselectivity, with DACH-ZYC-Phos- C1 as the best ligand. The synthetic potential of the C-allenylation products is demonstrated. Furthermore, the enantioselectivity observed with DACH-ZYC-Phos-C1 is rationalized by density functional theory studies. 1 Laboratory of Molecular Recognition and Synthesis, Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, P. R. China. 2 State Key Laboratory ✉ of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, P. R. China. email: [email protected]; [email protected] NATURE COMMUNICATIONS | (2021) 12:2416 | https://doi.org/10.1038/s41467-021-22498-1 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22498-1 yrazolones are a vital class of heterocycles possessing various with 84% ee (Fig. 2, entries 1–5). After optimizing the tempera- Pbiological properties1. Of particular interest are the pyr- ture and concentration (Fig. 2, entries 6–11), the reaction at 0.02 azolone derivatives with a chiral center (Fig.