Decision 2/8: Establishing a List of Hazardous Substances to Be Considered Hazardous Waste Under Article 2, Paragraph 1 (D)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Insecticide Use for Vector-Borne Disease Control
WHO/CDS/NTD/WHOPES/GCDPP/2007.2 GLOBAL INSECTICIDE USE FOR VECTOR-BORNE DISEASE CONTROL M. Zaim & P. Jambulingam DEPARTMENT OF CONTROL OF NEGLECTED TROPICAL DISEASES (NTD) WHO PESTICIDE EVALUATION SCHEME (WHOPES) First edition, 2002 Second edition, 2004 Third edition, 2007 © World Health Organization 2007 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. The named authors alone are responsible for the views expressed in this publication. CONTENTS Page Acknowledgements i Introduction 1 Collection of information 2 Data analysis and observations on reporting 3 All uses in vector control 6 Malaria vector control 22 Dengue vector control 38 Chagas disease vector control 48 Leishmaniasis vector control 52 Other vector-borne disease control 56 Selected insecticides – DDT 58 Selected insecticides – Insect growth regulators 60 Selected insecticides – Bacterial larvicides 62 Country examples 64 Annex 1. -
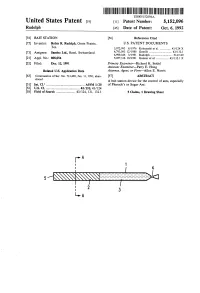
United States Patent (19) 11 Patent Number: 5,152,096 Rudolph 45 Date of Patent: Oct
III USOO5152096A United States Patent (19) 11 Patent Number: 5,152,096 Rudolph 45 Date of Patent: Oct. 6, 1992 (54) BAIT STATION (56) References Cited (75) Inventor: Robin R. Rudolph, Grain Prairie, U.S. PATENT DOCUMENTS Tex. 3,972,993 8/1976 Kobayashi et al................ 43/124 X 4,793,093 12/1988 Gentile ............................... 43/132.1 (73) Assignee: Sandoz Ltd., Basel, Switzerland 4,999,346 3/1991 Rudolph .............................. 514/120 21 Appl. No.: 808,054 5,057,316 10/1991 Gunner et al. ................. 43/132.1 X 22 Filed: Dec. 12, 1991 Primary Examiner-Richard K. Seidel Assistant Examiner-Patty E. Hong Related U.S. Application Data Attorney, Agent, or Firm-Allen E. Norris 63 Continuation of Ser. No. 713,480, Jun. 11, 1991, aban 57) ABSTRACT doned. A bait station device for the control of ants, especially (51) Int. Cl. ............................................... A0M 1/20 of Pharaoh's or Sugar Ant. (52 U.S. C. ......................................... 43/131; 43/124 58) Field of Search ....................... 43/124, 131, 132.1 5 Claims, 1 Drawing Sheet U.S. Patent Oct. 6, 1992 5,152,096 DSN a- 5,152,096 1. 2 enting the ants with a combination of an insect growth BAIT STATION regulant (IGR) bait and insecticide bait in such a way that the worker ants have to forage their way through This is a continuation of application Ser. No. the IGR bait to reach the insecticide bait. 07/713,480, filed Jun. 11, 1991, now abandoned. 5 In this way foraging worker ants will transport back The present invention concerns a bait station device to nests for feeding of the colony IGR bait and upon for the control of ants, especially of Pharaoh's or Sugar exhausting the available IGR bait will themselves ingest Ant. -

Vertebrate Pest Council 2002
UC Agriculture & Natural Resources Proceedings of the Vertebrate Pest Conference Title Rats! Foiled Again: A History of Rodent Control Methods Development at the National Wildlife Research Center Permalink https://escholarship.org/uc/item/3526b1nt Journal Proceedings of the Vertebrate Pest Conference, 25(25) ISSN 0507-6773 Authors Fagerstone, Kathleen A. Fall, Michael W. Witmer, Gary W. et al. Publication Date 2012 DOI 10.5070/V425110585 eScholarship.org Powered by the California Digital Library University of California Rats! Foiled Again: A History of Rodent Control Methods Development at the National Wildlife Research Center Kathleen A. Fagerstone, Michael W. Fall, and Gary W. Witmer USDA APHIS Wildlife Services, National Wildlife Research Center, Fort Collins, Colorado William C. Pitt USDA APHIS Wildlife Services, National Wildlife Research Center, Hawaii Field Station, Hilo, Hawaii ABSTRACT: The National Wildlife Research Center (NWRC) and its predecessor laboratories have a long history of developing ma- terials and methods for managing rodents and the damage they cause. The NWRC has been influential in exploring, developing, and maintaining legal uses of many traditional field rodenticides such as strychnine and zinc phosphide. Products have been developed for managing rodents in a variety of locales, and for managing a variety of species, from commensal rodents in urban areas, to pocket gophers and mountain beaver in forests, prairie dogs and ground squirrels on rangelands, and nutria and beaver in wetlands. Consid- erable research has also been conducted on developing methods of managing rodents in underdeveloped countries. Recent efforts by NWRC have focused on development of tools for managing invasive rodents in conservation areas such as island ecosystems and development of alternative, nonlethal control methods. -

Routine Multipesticide Analysis Orbitrap
Routine Multi-Pesticide Residue Analysis by Orbitrap MS Technology Osama Abu-Nimreh CMD Sales Support Specialist MECEC , Dubai The world leader in serving science Challenges of Pesticide-Residues Analysis • Sample variability (matrix) • Different compound characteristics • Large number of samples • Hundreds of analytes monitored • Low levels controlled • Baby food (MRL for all pesticides = 0.01 mg/kg) • Fast response required 2 Former Pesticide Multi-Residue Method Setup . Extraction Acetonitrile, Ethyl Mostly replaced acetate, Methanol... by QuEChERS today . Clean-up GPC, SPE, LLE, LC . Determination GC, LC, GC-MS, LC-MS, Thermo Scientific™ QuEChERS™ GC-MS/MS, LC-MS/MS... method 3 Simplified Extraction Procedure Applied 10 g of sample is weighed into Quechers extraction tube + 20 mL of water + 10 mL of ACN shaking 10 min Centrifugation 5 min @ 5000 rpm Injection to LC-HRAM 4 Consumables Used Consumables/Chemicals Part Number Acetonitrile A/0638/17 QuEChERS extraction tube, 50 mL, 250 pack 60105-216 QuEChERS pouches, 50 pack 60105-344 Apparatus/Columns Part Number Horizontal shaker 1069-3391 Horizontal shaker plate 1053-0102 Thermo Scientific™ Barnstead™ EASYpure™II water 3125753 Thermo Scientific™ Heraeus™ Fresco™ 17 micro centrifuge 3208590 Thermo Scientific™ Accucore™ aQ column 100x2.1, 2.6 µm 17326-102130 5 Improving QuEChERS Extraction Tips & Tricks: • Dry food (cereals/dried food, < 25 % water content): • Addition of water to enable adequate partitioning and reducing interaction of pesticides with matrix • Food containing fat/wax (avocado/oil): • After extraction step add a freezing out step and transfer supernatant to clean-up tube • More clean-up might be needed of raw extract (PSA+C18) • Food containing complex matrix (tea/spices) • Additional clean-up with GCB might be necessary (potential loss of planar structure pesticides like thiabendazole) • Acidic food (citrus): • Adjust pH (5-5.5) to increase recovery (e.g. -

COMBINED LIST of Particularly Hazardous Substances
COMBINED LIST of Particularly Hazardous Substances revised 2/4/2021 IARC list 1 are Carcinogenic to humans list compiled by Hector Acuna, UCSB IARC list Group 2A Probably carcinogenic to humans IARC list Group 2B Possibly carcinogenic to humans If any of the chemicals listed below are used in your research then complete a Standard Operating Procedure (SOP) for the product as described in the Chemical Hygiene Plan. Prop 65 known to cause cancer or reproductive toxicity Material(s) not on the list does not preclude one from completing an SOP. Other extremely toxic chemicals KNOWN Carcinogens from National Toxicology Program (NTP) or other high hazards will require the development of an SOP. Red= added in 2020 or status change Reasonably Anticipated NTP EPA Haz list COMBINED LIST of Particularly Hazardous Substances CAS Source from where the material is listed. 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide Acutely Toxic Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- Acutely Toxic 1-(2-Chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (Methyl-CCNU) Prop 65 KNOWN Carcinogens NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) IARC list Group 2A Reasonably Anticipated NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) (Lomustine) Prop 65 1-(o-Chlorophenyl)thiourea Acutely Toxic 1,1,1,2-Tetrachloroethane IARC list Group 2B 1,1,2,2-Tetrachloroethane Prop 65 IARC list Group 2B 1,1-Dichloro-2,2-bis(p -chloropheny)ethylene (DDE) Prop 65 1,1-Dichloroethane -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

B Commission Regulation (Eu)
02010R0037 — EN — 29.09.2018 — 035.001 — 1 This text is meant purely as a documentation tool and has no legal effect. The Union's institutions do not assume any liability for its contents. The authentic versions of the relevant acts, including their preambles, are those published in the Official Journal of the European Union and available in EUR-Lex. Those official texts are directly accessible through the links embedded in this document ►B COMMISSION REGULATION (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin (Text with EEA relevance) (OJ L 15, 20.1.2010, p. 1) Amended by: Official Journal No page date ►M1 Commission Regulation (EU) No 758/2010 of 24 August 2010 L 223 37 25.8.2010 ►M2 Commission Regulation (EU) No 759/2010 of 24 August 2010 L 223 39 25.8.2010 ►M3 Commission Regulation (EU) No 761/2010 of 25 August 2010 L 224 1 26.8.2010 ►M4 Commission Regulation (EU) No 890/2010 of 8 October 2010 L 266 1 9.10.2010 ►M5 Commission Regulation (EU) No 914/2010 of 12 October 2010 L 269 5 13.10.2010 ►M6 Commission Regulation (EU) No 362/2011 of 13 April 2011 L 100 26 14.4.2011 ►M7 Commission Regulation (EU) No 363/2011 of 13 April 2011 L 100 28 14.4.2011 ►M8 Commission Implementing Regulation (EU) No 84/2012 of 1 L 30 1 2.2.2012 February 2012 ►M9 Commission Implementing Regulation (EU) No 85/2012 of 1 L 30 4 2.2.2012 February 2012 ►M10 Commission Implementing Regulation (EU) No 86/2012 of 1 L 30 6 2.2.2012 February 2012 ►M11 Commission -

Comments of Teresa Homan with Attachments
Teresa Homan Watertown, SD 57201 I am a landowner in Deuel County, South Dakota. Our land boarders the Deuel Harvest Wind Project in Deuel county, Docket # EL 18-053. There are 112 towers cited in the project, with 9 towers within a mile of our property. We have spent over three decades developing this property to enhance wildlife and for the enjoyment of our family. Can you imagine how we felt when we found we have a population of eastern bluebirds? We have yellow warblers, which by the way feed on the web worms that form in our trees. We have orioles, cedar waxwings, brown thrashers, rose breasted grosbeaks, gold finches, purple finches, robins, blue jays, nuthatches, eastern kingbirds, bitterns, dark eyed juncos, red winged blackbirds, morning doves, owls, cow birds, northern mocking birds, grey cat birds, wood thrushes, tufted titmouse, king fishers, indigo buntings, scarlet tanagers, bobolinks, meadowlarks, many woodpeckers, turkeys, turkey vultures, even humming birds and bald eagles. There are more, just to numerous to list. Many of these birds we have seen for the first time in our lives on this property in the past 1 O years. Not only are these birds beautiful and fun to watch, they have their purpose in the ecosystem. We also see northern long eared bats, that are on the endangered list in South Dakota. These birds are making a come back after the use of insecticides that nearly wiped out many. In the 1940's the insecticide DDT was introduced for public use, it is now banned from sale. In 1976 the herbicide Roundup was introduced to the public. -

Comparison of Acute Noaels and Benchmark Doses for Female Brain Cholinesterase Inhibition
Supplemental Material for: February 5-8, 2002 SAP 25 January 2002 Comparison of Acute NOAELs and Benchmark Doses for Female Brain Cholinesterase Inhibition In cumulative risk assessment, it is important to characterize both the time frame for exposure (e.g., What is the exposure duration?) and for the toxic effect (e.g., What are the time to peak effects and the time to recovery?). In the Preliminary Cumulative Risk Assessment of the Organophoshate Pesticides (OPs) relative potency factors (RPFs) for 29 chemicals and points of departure (PODs) and the index chemical were determined based on whole brain cholinesterase (ChE) data from toxicity studies of 21 days and longer. The Office of Pesticide Programs has argued that the use of steady state data for relative potency determination generates relative potency factors (RPFs) that are reproducible and reflect less variability than RPFs derived from single-dose or short-term studies where the extent of inhibition changes rapidly immediately following dosing. OPP has posed a question to the FIFRA SAP for the February 5-8, 2002 review concerning how best to evaluate risk, taking into account the temporal characteristics of the hazard endpoint (i.e., cholinesterase inhibition) and the temporal characteristics of the exposure patterns for the food, drinking water, and residential/nonoccupational pathways. In order to facilitate the panel discussion, a table listing the available single dose toxicity studies performed with OPs has been made. Most of the studies are acute neurotoxicity (ACN) studies (OPPT Guideline 870.6200, OPP Guideline 81-8) administered by gavage. Acute lethality studies were not included. Dose levels, no- observed-adverse-effect levels (NOAELs), and no-observed-adverse-effect levels (LOAELs) for female brain ChE are also listed in the table. -

Giftfreies EUROPA Giftfreies Europa Giftfreies Europa
Hiltrud Breyer (Hrsg.) Dangerous chemicals Giftfreies EUROPA GIFTFREIES EUROPA Giftfreies Europa Herausgegeben von Hiltrud Breyer Die Grünen | Europäische Freie Allianz im Europäischen Parlament Für die sprachliche Gleichstellung von Männern und Frauen gilt: Die Entscheidung über gewählte Sprach- formen lag bei den AutorInnen. Die gewählte Sprachform ist jeweils weiblich und männlich zu verstehen. Das Werk einschließlich aller seiner Teile ist urheberrechtlich geschützt. Jede Verwertung, die nicht ausdrücklich vom Urheberrechtsgesetz zugelassen ist, bedarf der vorherigen Zustimmung. Giftfreies Europa Herausgegeben von Hiltrud Breyer Die Grünen | Europäische Freie Allianz im Europäischen Parlament Redaktion und Koordination: Stefan Ziller Redaktionelle Mitarbeit: Ilka Sille und Gasimi Nigar Ahmadaga Gestaltung: Matthias Roth und Stefan Ziller Druck: agit-druck Hilrdud Breyer, MdEP Rue Wiertz 60, B-1047 Brüssel, Belgien E [email protected] W www.hiltrudbreyer.eu Inhalt Einleitung Giftfreies Europa 9 Hiltrud Breyer Gifte im Alltag - unsere tägliche Gefahr Viele Kitas stark mit Weichmachern belastet 11 Sarah Häuser, Ann-Katrin Sporkmann Innenraum-Luft - Ein vernachlässigtes Thema 17 Hanns Moshammer Gift in Kleidung - Chemikalien in Textilien 25 Mecki Naschke Kontaminierte Kabinenluft in Passagierflugzeugen 31 Aida Infante Endocrine disruptors in the healthcare sector 41 Healthcare Without Harm Europe Chemische Zeitbomben 47 Philipp Mimkes Minimierung krebserzeugender Stoffe ein Ansatz zur Verminderung arbeitsbedingter Krebserkrankungen -

Report Name:Ukraine's Mrls for Veterinary Drugs
Voluntary Report – Voluntary - Public Distribution Date: November 05,2020 Report Number: UP2020-0051 Report Name: Ukraine's MRLs for Veterinary Drugs Country: Ukraine Post: Kyiv Report Category: FAIRS Subject Report Prepared By: Oleksandr Tarassevych Approved By: Robin Gray Report Highlights: Ukraine adopted several maximum residue levels (MRLs) for veterinary drugs, coccidiostats and histomonostats in food products of animal origin. Ukraine also adopted a list of drugs residues that are not allowed in food products. THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY The Office of Agricultural Affairs of USDA/Foreign Agricultural Service in Kyiv, Ukraine prepared this report for U.S. exporters of domestic food and agricultural products. While every possible care was taken in the preparation of this report, information provided may not be completely accurate either because policies have changed since the time this report was written, or because clear and consistent information about these policies was not available. It is highly recommended U.S. exporters verify the full set of import requirements with their foreign customers, who are normally best equipped to research such matters with local authorities, before any goods are shipped. This FAIRS Subject Report accompanies other reports on Maximum, Residue Limits established by Ukraine in 2020. Related reports could be found under the following links: 1.) Ukraine's MRLs for Microbiological Contaminants_Kyiv_Ukraine_04-27-2020 2.) Ukraine's MRLs for Certain Contaminants_Kyiv_Ukraine_03-06-2020 Food Products of animal origin and/or ingredients of animal origin are not permitted in the Ukrainian market if they contain certain veterinary drugs residues in excess of the maximum residue levels established in Tables 1 and 2. -

First Evidence of Anticoagulant Rodenticides in Fish in German
Environmental Science and Pollution Research https://doi.org/10.1007/s11356-018-1385-8 ADVANCEMENTS IN CHEMICAL METHODS FOR ENVIRONMENTAL RESEARCH First evidence of anticoagulant rodenticides in fish and suspended particulate matter: spatial and temporal distribution in German freshwater aquatic systems Matthias Kotthoff1 & Heinz Rüdel2 & Heinrich Jürling1 & Kevin Severin1 & Stephan Hennecke1 & Anton Friesen3 & Jan Koschorreck3 Received: 27 September 2017 /Accepted: 24 January 2018 # The Author(s) 2018. This article is an open access publication Abstract Anticoagulant rodenticides (ARs) have been used for decades for rodent control worldwide. Research on the exposure of the environment and accumulation of these active substances in biota has been focused on terrestrial food webs, but few data are available on the impact of ARs on aquatic systems and water organisms. To fill this gap, we analyzed liver samples of bream (Abramis brama) and co-located suspended particulate matter (SPM) from the German Environmental Specimen Bank (ESB). An appropriate method was developed for the determination of eight different ARs, including first- and second-generation ARs, in fish liver and SPM. Applying this method to bream liver samples from 17 and 18 sampling locations of the years 2011 and 2015, respectively, five ARs were found at levels above limits of quantifications (LOQs, 0.2 to 2 μgkg−1). For 2015, brodifacoum was detected in 88% of the samples with a maximum concentration of 12.5 μgkg−1. Moreover, difenacoum, bromadiolone, difethialone, and flocoumafen were detected in some samples above LOQ. In contrast, no first generation AR was detected in the ESB samples. In SPM, only bromadiolone could be detected in 56% of the samples at levels up to 9.24 μgkg−1.A temporal trend analysis of bream liver from two sampling locations over a period of up to 23 years revealed a significant trend for brodifacoum at one of the sampling locations.