790 Manuscript Info Abstract Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Current and Potential Use of Phytophagous Mites As Biological Control Agent of Weeds
Chapter 5 Current and Potential Use of Phytophagous Mites as Biological Control Agent of Weeds Carlos Vásquez, Yelitza Colmenárez, José Morales-Sánchez, Neicy Valera, María F. Sandoval and Diego Balza Additional information is available at the end of the chapter http://dx.doi.org/10.5772/59953 1. Introduction Biological control of weeds by using phytophagous mites may help to contain infestations and reduce their spread in time. Although, eradication is not the goal due to the vastness of the areas, the most desirable scenario is achieved when weeds are no longer a concern and no other control is necessary. However, biological control should not be considered the unique strategy to face weed problems, thus commonly; other methods are still required to attain the desired level of control. There is an increasingly interest in using mites for biological control of weeds, primarily those belonging to Eriophyidae because of they are host-specific and often weaken the host plant affecting plant growth and reproduction. Although eriophyid mite species impact the fitness of their host plant, it is not clear how much they have contributed to reduction of the population of the target weed. In some cases, natural enemies, resistant plant genotypes, and adverse abiotic conditions have reduced the ability of eriophyid mites to control target weed popula‐ tions. Besides, susceptibility of eriophyids to predators and pathogens may also prevent them from achieving population densities necessary to reduce host plant populations. In addition to eriophyid mites, tetranychid mites are also being considered as an alternative for weed control. The gorse spider mite, Tetranychus lintearius Dufour, has shown to reduce shoot growth on gorse (Ulex europaeus L.) by around 36% in impact studies conducted over 2.5 years in Tasmania. -

Insecta Mundi 0789: 1–56
InsectaA journal of world insect systematics Mundi 0789 A new genus and 37 new noctuoid species from peninsular Page Count: 56 Florida and the Keys (Lepidoptera: Noctuoidea) J. T. Troubridge 23396 Mullins Ave Port Charlotte, FL, U.S.A. 33954 Date of issue: September 25, 2020 Center for Systematic Entomology, Inc., Gainesville, FL Troubridge JT. 2020. A new genus and 37 new noctuoid species from peninsular Florida and the Keys (Lepidop- tera: Noctuoidea). Insecta Mundi 0789: 1–56. Published on September 25, 2020 by Center for Systematic Entomology, Inc. P.O. Box 141874 Gainesville, FL 32614-1874 USA http://centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non- marine arthropod. Topics considered for publication include systematics, taxonomy, nomenclature, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medi- cal entomology, pest control research, etc.), and no longer publishes book reviews or editorials. Insecta Mundi publishes original research or discoveries in an inexpensive and timely manner, distributing them free via open access on the internet on the date of publication. Insecta Mundi is referenced or abstracted by several sources, including the Zoological Record and CAB Abstracts. Insecta Mundi is published irregularly throughout the year, with completed manuscripts assigned an individual number. Manuscripts must be peer reviewed prior to submission, after which they are reviewed by the editorial board to ensure quality. One author of each submitted manuscript must be a current member of the Center for Systematic Entomology. Guidelines and requirements for the preparation of manuscripts are available on the Insecta Mundi website at http://centerforsystematicentomology.org/insectamundi/ Chief Editor: David Plotkin, [email protected] Assistant Editor: Paul E. -

Biological Control Investigations on Lantana
134 Proceedings, Hawaiian Entomological Society Biological Control Investigations on Lantana N. L. H. Krauss STATE DEPARTMENT OF AGRICULTURE HONOLULU, HAWAII (Submitted for publication December, 1961) Lantana {Lantana camara aculeata) is a tropical American shrub of the family Verbenaceae which has become a very serious pest of rangeland and other agricultural land in Hawaii, Fiji, Australia, Africa, and many other tropical regions. The science of biological control of weeds was begun by Albert Koebele, Government Entomologist of Hawaii, who in 1902 introduced more than 20 species of insects into Hawaii from Mexico against Lantana. Eight of these species were established in the islands and a fair degree of control of the plant was obtained in some areas, especially in the drier regions. In 1952 the author began work on this problem in Cuba, and since that year has carried on investi gations from time to time in various parts of tropical and subtropical America from California, Texas, and Florida to Argentina, and in other regions. Some of the insects found are briefly discussed in this paper. Selected insects were dispatched to Hawaii by air and the work of propagation, testing of food habits in a quarantine insectary, and liberation of approved species was carried on there by Q. C. Chock, C. J. Davis, P. W. Weber, H. K. Nakao, Mabel Chong, and other entomologists of the State Department of Agriculture (formerly Board of Agriculture and Forestry). A great deal of useful assistance was given me by entomologists in the countries visited. Most of the identifications of insects was done by specialists of the Entomology Research Division, U.S. -

Note Establishment of the Leaf Mining Fly, Calycomyza Lantanae Frick, On
Micronesica 30(2):417- 419, 1997 Note Establishment of the Leaf Mining Fly, Calycomyza lantanae Frick, on the weed Lantana camara L. on Pohnpei NELSON M. ESGUERRA, l<LASTHIN J. DIOPULOS, RODASIO P. SAMUEL, AND JONAH D. WILLIAM College of Micronesia - FSM P.O. Box 159, Kolonia Pohnpei FM 96941 Abstract-Despite a number of biological control agents released a few years ago to control Lantana camara, the weed persists in thickets, par ticularly along roadsides and open lands on Pohnpei. A leaf mining fly, Calycomyza lantanae, was introduced from Guam and established on three release sites on Pohnpei . Blotched mines on the leaves of L. camara become evident in the release sites. C. lantanae has spread throughout most of Pohnpei. Introduction Pohnpei is one of many western Pacific islands that has widespread growth of lantana, Lantana camara L., despite a number of biological control agents that have been released to control it. L. camara is an important weed pest along road sides and on range land, pasture and vacant lands . L. camara poses a serious long term threat to the vegetation on Pohnpei. A number of biological control agents were introduced to Pohnpei to control L. camara in 1948, between 1955 to 1958, in 1963, and in 1991 (Table I) (Es guerra et al. 1990, Schreiner 1989, Suta & Esguerra 1993). Despite the release of a number of biological control agents, L. camara is still widespread throughout the island. A request was made to Dr. R. Muniappan, Uni versity of Guam College of Agriculture and Life Sciences to send us the leaf min ing fly, Calycomyza lantanae Frick, to further suppress L. -
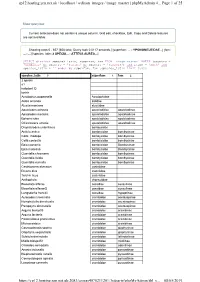
Page 1 of 25 Cp12.Hosting.Zen.Net.Uk / Localhost / Wdixon Images / Image Master | Phpmyadmin 4... 08/05/2019
cp12.hosting.zen.net.uk / localhost / wdixon_images / image_master | phpMyAdmin 4... Page 1 of 25 Show query box Current selection does not contain a unique column. Grid edit, checkbox, Edit, Copy and Delete features are not available. Showing rows 0 - 657 (658 total, Query took 0.0117 seconds.) [superfam: ... - YPONOMEUTIDAE...] [fam: ... - ...] [species_latin: 2 SPECIS... - ATTEVA AUREA...] SELECT distinct species_latin, superfam, fam FROM `image_master` WHERE (country = 'Honduras' or country = 'Panama' or country = 'Pan2018') and itype = 'moth' and species_latin > '' order by superfam, fam ,species_latin limit 0,850 species_latin 3 superfam 1 fam 2 2 specis a1 notodont Q tortrix Acrolophus popeanella Acrolophidae Aidos amanda aididae Alucita montana alucitidae Apatelodes adrastia apatelodidae apatelodinae Apatelodes merlona apatelodidae apatelodinae Ephoria lybia apatelodidae apatelodinae Olceclostera amoria apatelodidae apatelodinae Drepatelodes umbrillinea bombycidae Anticla antica bombycidae bombycinae Colla rhodope bombycidae bombycinae Colla coelestis bombycidae bombycinae Epia casnonia bombycidae Bombycinae Epia muscosa bombycidae Bombycinae Quentalia chromana bombycidae bombycinae Quentalia lividia bombycidae bombycinae Quentalia numalia bombycidae bombycinae Castniomera atymnius castniidae Divana diva castniidae Telchin licus castniidae Anthophyla choreutidae Biocellata alfarae cossidae cossulinae Biocellata alfaraeQ cossidae cossulinae Langsdorfia franckii cossidae hypoptinae Aulacodes traversalis crambidae acentropinae Nymphuliella -

Review and Evaluation of Lantana Biocontrol Programs
Biological Control 17, 272–286 (2000) doi:10.1006/bcon.1999.0793, available online at http://www.idealibrary.com on COMMENTARY Review and Evaluation of Lantana Biocontrol Programs Sonya Broughton1 Department of Entomology and Zoology, The University of Queensland, St Lucia, Queensland 4072, Australia Received February 16, 1999; accepted October 8, 1999 feeding insect species were identified as successful This paper provides a review of lantana (Lantana agents, but different species have been successful in camara L.) biological control programs worldwide. different countries (Crawley, 1986, 1989a,b). Tables on the origins of the agents introduced for the Inability to predict success is attributed to the low biocontrol of lantana, are presented, including refer- genetic uniformity of lantana and its ability to colonize ences to the biology and/or host-tests for each species. diverse habitats (Crawley, 1989a,b; Willson, 1993; Swar- Establishment and control rates of the introduced agents and cases leading to partial control of lantana brick et al., 1995). Because of this, Willson (1993) are discussed. From the review, feeding groups and suggested that agent selection procedures proposed by species contributing to control were identified. Leaf-, Harris (1973a,b), Goeden (1983), and Wapshere (1985) flower-, and fruit-feeding species were the most success- are irrelevant. Instead, species were prioritized accord- ful feeding groups, and the leaf-mining chrysomelid, ing to their success in other countries or because they Uroplata girardi Pic, was the most successful control were host-specific and added to the folivore complex agent. The main factor preventing establishment was already introduced (Harley and Kassulke, 1971; Water- the number of individuals released, while cultivar house and Norris, 1987; Willson, 1993). -

An Annotated List of the Lepidoptera of Honduras
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 2-29-2012 An annotated list of the Lepidoptera of Honduras Jacqueline Y. Miller University of Florida, [email protected] Deborah L. Matthews University of Florida, [email protected] Andrew D. Warren University of Florida, [email protected] M. Alma Solis Systematic Entomology Laboratory, PSI, Agriculture Research Service, USDA, [email protected] Donald J. Harvey Smithsonian Institution, Washington, D.C., [email protected] See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Miller, Jacqueline Y.; Matthews, Deborah L.; Warren, Andrew D.; Solis, M. Alma; Harvey, Donald J.; Gentili- Poole, Patricia; Lehman, Robert; Emmel, Thomas C.; and Covell, Charles V., "An annotated list of the Lepidoptera of Honduras" (2012). Insecta Mundi. 725. https://digitalcommons.unl.edu/insectamundi/725 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Jacqueline Y. Miller, Deborah L. Matthews, Andrew D. Warren, M. Alma Solis, Donald J. Harvey, Patricia Gentili-Poole, Robert Lehman, Thomas C. Emmel, and Charles V. Covell This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ insectamundi/725 INSECTA A Journal of World Insect Systematics MUNDI 0205 An annotated list of the Lepidoptera of Honduras Jacqueline Y. Miller, Deborah L. -

Evaluating Prospects for Biological Control of Invasive Weeds In
Evaluating prospects for biological control of invasive weeds in Melanesia: A report on the ACIAR-funded workshop held at the Novotel Hotel, Nadi, Fiji, 25-27th November 2013 Michael Day Senior Entomologist Biosecurity Queensland Department of Agriculture, Fisheries and Forestry Ecosciences Precinct GPO Box 267, Brisbane, 4001 Qld [email protected] A workshop was held at the Novotel Hotel, Nadi, Fiji from 25-27th November 2013 to prioritise weeds that could be included in a possible future ACIAR-funded regional biocontrol project (Appendix 1). It was attended by 18 people, representing 12 organisations in eight countries. Caress Whippy and Joeli Uluinayau, Secretariat of the Pacific Community (SPC) provided valuable logistical support (Appendix 2). Day 1 Wednesday, 25th November 2013 The meeting was opened by Tony Gunua, SPC, followed by an address by Richard Markham, ACIAR Program Manager, Pacific Crops. Participants were asked what they can contribute to the workshop, as well as what they hoped they could gain from the workshop. Most thought they appreciated the opportunity to get together with researchers from other countries and organisations in the region and have the opportunity to provide input into developing a regional biocontrol program. Dick Watling, Nature Fiji Mareqeti Viti, gave a presentation on the perceptions of Spathodea campanulata. He presented a case that the species was not invasive and not having any impact on biodiversity, forestry or agriculture. He said that there has not been any research conducted to quantify impacts of this species, positive or negative. Therefore, before a decision on biocontrol of African tulip tree is made, research should be conducted to understand the role of this species in the landscape. -

JGF Genitalia Slide Collection
John G. Franclemont genitalia slide collection Cornell University Insect Collection A B C DE 1 JGF Slide No. Genus species Author sex 2 4517 Abagrotis alampeta m 3 4518 Abagrotis alampeta f 4 4519 Abagrotis alampeta m 5 4520 Abagrotis alampeta f 6 4521 Abagrotis alampeta f 7 546 Abagrotis alternata m 8 556 Abagrotis alternata m 9 558 Abagrotis alternata m 10 1656 Abagrotis alternata m 11 3403 Abagrotis alternata m 12 7301 Abagrotis alternata m 13 4513 Abagrotis barnesi m 14 4514 Abagrotis barnesi f 15 4515 Abagrotis barnesi m 16 4531 Abagrotis barnesi m 17 7627 Abagrotis crumbi m 18 7628 Abagrotis crumbi m 19 7617 Abagrotis erratica m 20 7618 Abagrotis erratica f 21 5001 Abagrotis placida m 22 5002 Abagrotis placida f 23 4516 Abagrotis reedi (=nefascia auct) m 24 5003 Abagrotis reedi (=nefascia auct) m 25 5004 Abagrotis reedi (=nefascia auct) m 26 5005 Abagrotis reedi (=nefascia auct) f 27 5007 Abagrotis reedi (=nefascia auct) m 28 5006 Abagrotis scopeops m 29 5504 Abagrotis scopeops f 30 5505 Abagrotis scopeops m 31 5506 Abagrotis scopeops f 32 5507 Abagrotis scopeops m 33 5508 Abagrotis scopeops f 34 7615 Abagrotis trigona m 35 7616 Abagrotis trigona f 36 7625 Abagrotis trigona m 37 5502 Abagrotis m 38 5503 Abagrotis f 39 5510 Abagrotis f 40 7619 Abagrotis m 41 7620 Abagrotis f 42 7621 Abagrotis m 43 7622 Abagrotis f 44 7623 Abagrotis m 45 7624 Abagrotis m 46 7626 Abagrotis m 47 618 Abbottana clemataria m 48 2407 Abbottana clemataria m 49 939 Abrostola ovalis m 50 795 Abrostola urentis m 51 2368 Abrostola urentis m 52 2369 Abrostola urentis m 53 7381 Absideridis n. -
The First Introduction for Field Release Was. Mecas, Some 22,000 Roots Containing Larvae Being Collected
8 (b) - 7 The first introduction for field release was. Mecas, some 22,000 roots containing larvae being collected. in 1961:in.the southern United States of,America by Mr. J. Mann of the Queensland Lands Department. Beetles Commenced emerging .from these roots 15 months later, in December 1962, and since that time small liberations have been made at four points- Samford, Roma,. Biloela, and Rockhampton; oviposition and subsequent larval development, was found at Samford and at Roma. These insects are now being cage -bred each summer and it is hoped that liberations can be continued until establishment is achieved. Supplies of Nupserha were collected in India in..1963, again by Mr. J.. Mann. A total of 33,000 roots containing larvae were received. Emergence of these beetles, commenced in October 1964, and liberations were made at Rockhampton, Barcaldine, and other points in western Queensland. Cage- breeding to ensure supplies for further.liberations is also being attempted with this insect. Mann, J. Department'of Lands, Queensland INTRODUCTION OF DIASTEMA TIGRIS.GUENEE FOR LANTANA CONTROL Diastema tigris Guen. (Lepidoptera - Noctuidae) is a lantana foliage feeding moth native to North-and Central America and some of the Islands of the West Indies off the East Coast of America. It was recorded by Krauss and Mann during their 1953 survey of the insects of lantana in North and Central America. The insect was introduced into Hawaii from the Panama Canal Zone in 1954 for the biological control of lantana and, after a series of host feeding tests was satisfactorily concluded, it was liberated in several places. -
Insecta, Lepidoptera)
A peer-reviewed open-access journal ZooKeys 788:Additions 241–252 (2018) and corrections to the check list of the Noctuoidea (Insecta, Lepidoptera)... 241 doi: 10.3897/zookeys.788.28500 DATA PAPER http://zookeys.pensoft.net Launched to accelerate biodiversity research Additions and corrections to the check list of the Noctuoidea (Insecta, Lepidoptera) of North America north of Mexico IV B. Christian Schmidt1, J. Donald Lafontaine1, James T. Troubridge2 1 Canadian National Collection of Insects, Arachnids, and Nematodes, Biodiversity Program, Agriculture and Agri-Food Canada, K.W. Neatby Bldg., 960 Carling Ave., Ottawa, Ontario, Canada K1A 0C6 2 Hagersville, Ontario, Canada Corresponding authors: B.C. Schmidt ([email protected]); J.D. Lafontaine ([email protected]) Academic editor: J. Adams | Received 19 July 2018 | Accepted 12 September 2018 | Published 8 October 2018 http://zoobank.org/8A6522C4-1220-4D31-869D-615410DC594A Citation: Schmidt BC, Lafontaine JD, Troubridge JT (2018) Additions and corrections to the check list of the Noctuoidea (Insecta, Lepidoptera) of North America north of Mexico IV. In: Schmidt BC, Lafontaine JD (Eds) Contributions to the systematics of New World macro-moths VII. ZooKeys 788: 241–252. https://doi.org/10.3897/ zookeys.788.28500 Abstract A summary of all taxonomic and nomenclatural changes to the check list of the Noctuoidea of North America north of Mexico since the last update published in 2015 is provided. A total of 64 changes are listed and discussed, consisting of 26 recently published changes and additions, and an additional 38 presented herein. One stat. n., one stat. rev., six syn. -

MOTHS of PANAMA X by Dr Bill Dixon Prepared for the Canopy Tower Page 1 Scientific Name Common Name Pan Ghost Moths Yucca Moths
MOTHS of PANAMA by Dr Bill Dixon prepared for the Canopy Tower x Scientific Name Common Name pan hon cuba braz conf% weblink localink Family Hepialidae Ghost moths Family Nepticulidae Nepticulids Family Opostegidae Opostegids Family Prodoxidae Yucca moths Family Tineidae Cloths moths Family Acrolophidae Burrowing webworms Family Psychidae Bagworm moths Family Gracilliariidae Leaf-blotch miners Family Bucculatricidae Ribbed cocoon makers Family Yponomeutidae Ermine moths Atteva aurea Ailanthus Webworm 1 http://mysqlautofindandmatch.com/searchtech3.php?ck1=all&ck2=moth&ck3=all&ck4=all&ck5=all&ck6=all&ck7=all&ck8=all&ck9=AttevaC:\Users\billdixon\Pictures\Camera Roll\camerafiles_2015\Honduras\SA-database-honduras\atteva-DSCN0965.jpg aurea&ck10=&ck11=all Family Plutellidae Diamondbacked moths Family Glyphipterigidae Sedge moths Family Bedelliidae Bedelliids Family Elachistidae Elachistids Family Autostichidae Autostichids Family Glyphidoceridae Glyphidocerids Family Batrachedridae Batrachedrids Family Oecophoridae Oecophorids Family Coleophoridae Case-bearers Family Cosmopterigidae Cosmopterigids Family Scythrididae gelechoideaea Family Gelechiidae Gelechiids Subfamily Gelechiinae Fascista cercerisella 1 http://mysqlautofindandmatch.com/searchtech3.php?ck1=all&ck2=moth&ck3=all&ck4=all&ck5=all&ck6=all&ck7=all&ck8=all&ck9=Fascista cercerisella&ck10=&ck11=all Subfamily Anacampsinae Anacampsis coverdalella 1 http://www.mysqlautofindandmatch.com/searchtech3.php?ck1=all&ck2=moth&ck3=all&ck4=all&ck5=all&ck6=all&ck7=all&ck8=all&ck9=Anacampsis coverdalella&ck10=&ck11=all