Interaction of Components in Aquatic System with the Chlorates and Chlorides Calcium, Magnesium and Acetatemonoethanolammonium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemistry of Natural Resources *OCE/26193* Candidates Answer on the Question Paper
ADVANCED SUBSIDIARY GCE CHEMISTRY B (SALTERS) F332/TEST Chemistry of Natural Resources *OCE/26193* Candidates answer on the question paper. Friday 27 May 2011 Afternoon OCR supplied materials: • Data Sheet for Chemistry B (Salters) (inserted) Duration: 1 hour 45 minutes • Advance Notice: ‘Polymers on the move’ (inserted) Other materials required: • Scientific Calculator *F332TEST* INSTRUCTIONS TO CANDIDATES • The insert will be found in the centre of this document. • Write your name, centre number and candidate number in the boxes above. Please write clearly and in capital letters. • Use black ink. Pencil may be used for graphs and diagrams only. • Read each question carefully. Make sure you know what you have to do before starting your answer. • Write your answer to each question in the space provided. If additional space is required, you should use the lined pages at the end of this booklet. The question number(s) must be clearly shown. • Answer all the questions. • Do not write in the bar codes. INFORMATION FOR CANDIDATES • The number of marks is given in brackets [ ] at the end of each question or part question. • Where you see this icon you will be awarded marks for the quality of written communication in your answer. This means for example you should: • ensure that text is legible and that spelling, punctuation and grammar are accurate so that meaning is clear; • organise information clearly and coherently, using specialist vocabulary when appropriate. • You may use a scientific calculator. • The insert ‘Polymers on the move’ is provided for use with question 5. • A copy of the Data Sheet for Chemistry B (Salters) is provided as an insert with this question paper. -

Kinetics of the Process of Conversion of Production Of
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 07, 2020 Kinetics Of The Process Of Conversion Of Production Of Calcium Chlorate With The Use Of A Filtrate Of Hydrocaldic Acid Decomposition Of Washed Ck Phosphonic Concentrate Khamdamova Shohida Sherzodovna1, Rozikova Dilshoda Abdullajanovna2 1Doctor of Technical Sciences Associate Professor of the Department of Chemical Technology, Fergana Polytechnic Institute, Fergana Uzbekistan 2Basic doctoral student of the Namangan Engineering and Technological Institute, Namangan, Uzbekistan Abstract: The process of conversion of 20-25% solutions of calcium chloride obtained by decomposition of phosphorites of Central Kizikumes with sodium chlorate at 50, 75, 100 and 125 ° C with evaporation and without evaporation of conversion solutions has been studied. The process activation energies, the order and the rate constant of the conversion reaction as well as kinetic values of the reagent consumption are determined depending on temperature, time and carrying conditions of processes. The order of the conversion process of calcium chloride with sodium chlorate equals one. It is confirmed by the fact that the conversion rate constant calculated on the basis of experimental data remains practically constant for each temperature. Also, the linear dependence of lg (C0-Cτ) on τ also indicates the first order of the conversion process of calcium chloride with sodium chlorate. The rate constant of conversion increases with a rise in temperature. Its dependence on temperature obeys Arrhenius law. To establish values of the conversion rate constant for different temperatures, the constants (К0) for 20 and 25% solutions of calcium chloride have been calculated by Arrhenius equation and the dependence equation of lg K on 1 / T has been derived. -

Six-Year Review 3 Technical Support Document for Chlorate
Six-Year Review 3 Technical Support Document for Chlorate Office of Water (4607M) EPA-810-R-16-013 December 2016 Disclaimer This document is not a regulation. It is not legally enforceable, and does not confer legal rights or impose legal obligations on any party, including EPA, states, or the regulated community. While EPA has made every effort to ensure the accuracy of any references to statutory or regulatory requirements, the obligations of the interested stakeholders are determined by statutes, regulations or other legally binding requirements, not this document. In the event of a conflict between the information in this document and any statute or regulation, this document would not be controlling. This page intentionally left blank. Table of Contents 1 Introduction ................................................................................................................. 1-1 2 Contaminant Background .......................................................................................... 2-1 2.1 Chemical and Physical Properties ................................................................................. 2-1 2.2 Production, Use and Release ......................................................................................... 2-2 2.2.1 Commercial Production and Use in Industry and Agriculture ........................... 2-2 2.2.2 Incidental Production and Release ...................................................................... 2-6 2.3 Environmental Fate ...................................................................................................... -
Material Safety Data Sheet
MATERIAL SAFETY DATA SHEET CALCIUM HYPOCHLORITE, SOLID 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Brenntag Canada Inc. WHMIS#: 00020005 43 Jutland Rd. Index: GCD0313/15D Toronto, ON Effective Date: 2015 October 02 M8Z 2G6 (416) 259-8231 Date of Revision: 2015 October 02 Website: http://www.brenntag.ca EMERGENCY TELEPHONE NUMBER (For Emergencies Involving Chemical Spills or Releases) 1 855 273 6824 PRODUCT IDENTIFICATION Product Name: Calcium Hypochlorite, Solid. Chemical Name: Hypochlorous acid, calcium salt. Synonyms: HTH Dry Chlorine Granular; Calcium Hypochlorite Granular; Calcium Hypo; Calcium Hypo Pitchl Gran 65%; Cal Hypo Comm 65% Tabs; Calcium Hypo PPG 3" Tabs; Accu-Tab White, Blue, SI, Blue SI, White SI Tabs. Chemical Family: Hypochlorous acid salt. Molecular Formula: Ca (ClO) 2. Product Use: Swimming pool water disinfectant. Water treatment. Oxidizing agent. Chemical intermediate. WHMIS Classification / Symbol: C: Oxidizer E: Corrosive READ THE ENTIRE MSDS FOR THE COMPLETE HAZARD EVALUATION OF THIS PRODUCT. 2. COMPOSITION, INFORMATION ON INGREDIENTS (Not Intended As Specifications) Ingredient CAS# ACGIH TLV (TWA) % Concentration Calcium Hypochlorite 7778-54-3 --- 60 - 80 Sodium Chloride 7647-14-5 --- 10 - 30 Calcium Carbonate 471-34-1 --- 1 - 5 Calcium Hydroxide 1305-62-0 5 mg/m³ 1 - 5 Calcium Chlorate 10137-74-3 --- 0 - 3 3. HAZARDS IDENTIFICATION Calcium Hypochlorite, Solid Brenntag Canada Inc. WHMIS Number : 00020005 Date of Revision: 2015 October 02 Page 2 of 9 EMERGENCY OVERVIEW: Corrosive! Causes severe skin and eye burns. Dust is extremely irritating to respiratory tract. See "Other Health Effects" Section. Oxidizing material. Contact with other combustible material can cause fire. This material is a strong oxidizer which is stable under normal conditions, but can decompose if contaminated. -

Ap Chemistry Summer Assignment
AP CHEMISTRY SUMMER ASSIGNMENT For: Students enrolled in 2018-2019 AP Chemistry Course From: Mrs. Vanessa Urteaga (L-154) Google Classroom Code: 239n0q This assignment is a review of things you should have mastered in Chemistry I or Pre-AP Chemistry. This assignment will be collected for a grade with the first half of it due July 20th, 2018 and the second half due August 17th, 2018. We will spend the first couple weeks of school to review prior content. If you have any questions please do not hesitate to contact me at the email address [email protected]. Welcome to AP Chemistry! I am very excited to have you enrolled in my class and cannot wait to get started! AP chemistry is a difficult course, but with some motivation and determination we will succeed! I am assigning a summer assignment because I need you ready for the start of the school year by reviewing the things you should have learned in Chemistry I or Pre-AP Chemistry. Remember this course is designed to match a first year college chemistry class…(YAY!!) I am extremely excited and I am sure we will have a fantastic year in AP Chemistry. (The summer assignment can also be found on the South Grand Prairie High School webpage, select Departments and Chemistry.) SUMMER ASSIGNMENT This summer assignment consists of worksheets and rules to memorize. There are three (3) worksheets total. • Worksheet #1 and #2 are due July 20th, 2018 via Google Classroom (take pic & upload). • Worksheet #3 is due August 17th, 2018 via Google Classroom (take a pic and upload). -

Interagency Committee on Chemical Management
DECEMBER 14, 2018 INTERAGENCY COMMITTEE ON CHEMICAL MANAGEMENT EXECUTIVE ORDER NO. 13-17 REPORT TO THE GOVERNOR WALKE, PETER Table of Contents Executive Summary ...................................................................................................................... 2 I. Introduction .......................................................................................................................... 3 II. Recommended Statutory Amendments or Regulatory Changes to Existing Recordkeeping and Reporting Requirements that are Required to Facilitate Assessment of Risks to Human Health and the Environment Posed by Chemical Use in the State ............................................................................................................................ 5 III. Summary of Chemical Use in the State Based on Reported Chemical Inventories....... 8 IV. Summary of Identified Risks to Human Health and the Environment from Reported Chemical Inventories ........................................................................................................... 9 V. Summary of any change under Federal Statute or Rule affecting the Regulation of Chemicals in the State ....................................................................................................... 12 VI. Recommended Legislative or Regulatory Action to Reduce Risks to Human Health and the Environment from Regulated and Unregulated Chemicals of Emerging Concern .............................................................................................................................. -

Corrosion Resistance
Rigid Nonmetallic Conduit – Corrosion Resistance Prime Conduit® Schedule 40 and Schedule 80 are generally acceptable for use in environments containing the chemicals below. These environmental resistance ratings are based upon tests where the specimens were placed in complete submergence in the reagent listed. Schedule 40 and Schedule 80 can be used in many process areas where chemicals not on this list are manufactured or used because worker safety requirements dictate that any air presence or splashing be at a very low level. If there are any questions for specific suitability in a given environment, prototype samples should be tested under actual conditions. Acetic Acid O-20% Butyl Alcohol Fluorine Gas – Wet Mercurous Nitrate Sodium Arsenite Acetic Acid 20-30% Butyl Phenol Fluorine Gas – Dry Mercury Sodium Benzoate Acetic Acid 3O-60% Butylene Fluoroboric Acid Methyl Sulfate Sodium Bicarbonate Acetic Acid 80% Butyric Acid Fluorosilicic Acid Methylene Chloride Sodium Bisulfate Acetic Acid – Glacial Calcium Bisulfite Formaldehyde Mineral Oils Sodium Bisulfite Acetic Acid Vapors Calcium Carbonate Formic Acid Naphthalene Sodium Bromide Acetylene Calcium Chlorate Fructose Nickel Chloride Sodium Chlorate Adipic Acid Calcium Chloride Gallic Acid Nickel Nitrate Sodium Chloride Alum Calcium Hydroxide Gas – Coke Oven Nitric Acid, Anydrous Sodium Cyanide Aluminum Chloride Calcium Hypochlorite Gas – Natural (Dry) Nitric Acid 20% Sodium Dichromate Aluminum Fluoride Calcium Nitrate Gas – Natural (Wet) Nitric Acid 40% Sodium Ferricyanide Aluminum -

2016-2017 AP Chemistry-Summer Assignment Students
2016-2017 AP Chemistry-Summer Assignment Students, Congratulations on embarking on this exciting journey through AP Chemistry. Most of you have a strong background in foundational techniques from your Chemistry 1 class, but AP Chemistry is a different beast! Understanding the material (as opposed to memorizing the night before the test) and creating a successful plan of attack is crucial, as you will be asked to apply your knowledge to many unknowns. The days of spitting back information are behind you! AP Chemistry is a difficult course. To succeed, you must keep up with the assignments and be willing to spend time working through the material. The purpose of this summer assignment will be make sure that everyone is at the same starting point come late August. It is due the first day of class and we will have a quiz on the material that is to be memorized the first week of school. The assignment will count as one test grade. I check my e-mail frequently, so feel free to contact me if you are having problems doing the summer assignment. Please take the summer assignment seriously. We will use the National Math and Science Initiative throughout the year. The following link will provide copies of essential information (notes, videos, answers, PT, and formula chart). Go there now! APChemistryNMSI - AP Chemistry Class Lecture Notes AND instructional videos 1. Print the official periodic table and formula chart. 2. Download the following notes: a. 01 Chemical Foundations b. 02 Atoms, Ions, and Molecules c. 03 Stoichiometry 3. Watch the companion videos and take notes. -

VANTAGE Calcium Hypochlorite Tablets
MATERIAL SAFETY DATA SHEET I - PRODUCT IDENTIFICATION Product: Calcium Hypochlorite Tablets Chemical Family: Hypochlorite Formula: Not Applicable / Mixture CAS Number: 7778-54-3 Synonyms: Cal Hypo Tabs COMPANY IDENTIFICATION 24 HR EMERGENCY TELEPHONE NUMBER AllChem Performance Products, LP INFOTRAC (Transportation): (800)535-5053 6010 NW First Place Gainesville, FL 32607 Tel: 352-378-9696 II – COMPOSITION, INFORMATION ON INGREDIENTS Available chlorine: 67% Exposure Limits Chemical or Common Name: OSHA PEL: ACGIH TLV: Calcium Hypochlorite 7778-54-3 60-80% Sodium Chloride 7647-14-5 10-20% NE NE Calcium Chlorate 10137-74-3 0-5% NE NE Calcium Chloride 10043-52-4 0-5% NE NE Calcium Hydroxide 1305-62-0 0-4% None 5mg/m3 Calcium Carbonate 471-34-1 0-5% 10mg/m3 15mg/m3 (Total Dust) 5mg/m3 (Respirable fraction) Water 7732-18-5 5.5-10% NE NE *NE- none established Calcium hypochlorite, calcium chlorate, calcium chloride, calcium hydroxide and calcium carbonate are hazardous per 29 CFR 1910.1200 III – HAZARDS IDENTIFICATION WARNING STATEMENT AND WARNING PROPERTIES: May be fatal if swallowed. Avoid breathing dust or fumes. Harmful if product is inhaled in high concentrations. Causes skin, eye, digestive tract and respiratory tract burns. Primary Route(s) of Entry: Ingestion: (X) Inhalation: (X) Skin Contact: (X) Eye Contact: (X) Primary Health Hazards (Acute and Chronic): Human Response Data: Odor Threshold: Approximately 1.4 mg/cubic-meter, based on odor threshold of chlorine. Irritation Threshold: Approximately 13-22 mg/cubic-meter, based on the irritation threshold of chlorine. 1 of 6 MATERIAL SAFETY DATA SHEET Immediately Dangerous to Life or Health: Approximately 45 mg/cubic-meter, based on IDLH Concentration of chlorine. -

Calcium Hypochlorite Granular Safety Data Sheet
Calcium Hypochlorite Granular Safety Data Sheet I Section 1: Identification Product identifier Product Name l Calcium Hypochlorite Granular Synonyms l AllClear™ ChlorRight; AllClear™ Shock Clear; AmeriChlor Calcium Hypochlorite Granules; Assalt 73; BioGuard Burn Out 73; BioGuard CLC Classic; Ca(OCl)2; Cal Hypo Granules; Calcium Hypochlorite; Calcium Hypochlorite Granular; Ideal Pool Products Super Shock 73; Induclor™; Induclor™ 70; Nature’s Way Super Pool Shock; Pittclor 70; Pittclor®; Power Powder® Plus™; Power Powder® Pro™; Prestochlor™; Pro Team Power 73; ProGuard; Refresh Dry Chlorinating Granular; Re Fresh®; Regal®; Repak™ + Granules; Repak™ Dry Chlorinating Granules; Super Pool Shock; Super ShockIt®; Super ShockIt® 73; Super Zappit™; Sustain® Shock Treatment; Vanguard® Plus Calcium Hypochlorite Granules; Zappit™; Zappit™ 73 Relevant identified uses of the substance or mixture and uses advised against Recommended use l Industrial Application, Chlorine Disinfectant, Pool Chemicals Details of the supplier of the safety data sheet Manufacturer l Axiall, LLC 1000 Abernathy Rd. NE, Suite 1200 Atlanta, GA 30328 United States www.axiall.com [email protected] Telephone (General) l +1 2256851240 Emergency telephone number Manufacturer l +1 3044556882 Section 2: Hazard Identification United States (US) According to: OSHA 29 CFR 1910.1200 HCS Classification of the substance or mixture OSHA HCS 2012 l Oxidizing Solids 2 Acute Toxicity Oral 4 Skin Corrosion 1B Serious Eye Damage 1 Specific Target Organ Toxicity Single Exposure 3: Respiratory Tract Irritation Label elements OSHA HCS 2012 DANGER Preparation Date: 30/March/2015 Format: GHS Language: English (US) Revision Date: 30/March/2015 WHMIS, OSHA HCS 2012 Page 1 of 13 Calcium Hypochlorite Granular Hazard statements l May intensify fire; oxidizer Harmful if swallowed Causes severe skin burns and eye damage. -

Summer Assignment for AP Chemistry: I Hope You Are All Ready for a Fun, Yet Challenging Year
Summer Assignment for AP Chemistry: I hope you are all ready for a fun, yet challenging year. You have a good foundation in basic chemistry from Chem 1, but AP Chem will be a little different. Rather than just memorizing how to do particular types of problems, you must instead really understand the chemistry behind each process and be able to apply it to all sorts of different situations. Students who finish AP Chemistry come out with a better understanding of the world around them. They also come out with a sense of accomplishment. It is a difficult class, but with determination and perseverance, you will surely succeed. Like almost all AP level courses, this course comes with a summer assignment. This assignment is strongly recommended, but not a requirement. It will undoubtedly help you as we dive deeper, but you are not going to get a grade for the completion of the assignment. I will assume though that you are proficient at these concepts. Start out the year on a good note. If you need help with the assignment, you will find it in your Chem 1 notes or on the internet. You may also feel free to work together with other students enrolled in the class. The assignment is designed to force you to review topics covered in Chem 1 that I expect you to be familiar with and having retained. Those topics include: Naming and writing formulas for ionic compounds and acids Empirical and Molecular formula calculations Stoichiometric calculations Balancing reaction equations Predicting products of the basic types of reactions We will begin the year with a brief project that will very quickly review the material listed above, and then test on that material. -
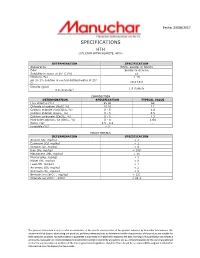
Specifications Hth (Calcium Hypochlorite, 68%)
Fecha: 23/08/2017 SPECIFICATIONS HTH (CALCIUM HYPOCHLORITE, 68%) DETERMINATION SPECIFICATION Appearance White, powder or tablets Odor Similar to chlorine Solubility in water at 25° C (%) 18 Moisture (%) < 16 pH (in 1% solution in neutral distilled water at 25° 10.4 10.8 C) Density (g/cc) 1.9 (tablets 0.8 (granular) COMPOSITION DETERMINATION SPECIFICATION TYPICAL VALUE Free chlorine (%) 65 80 68 Chloride of sodium (NaCl, %) 10 20 17 Calcium chlorate (Ca(ClO3)2, %) 0 - 5 1.4 Calcium chloride (CaCo3, %) 0 - 5 0.5 Calcium carbonate (CaCO3, %) 0 - 5 2.3 Hydroxide calcium (Ca (OH)2 , %) 0 - 4 1.64 Water (%) 5.5 - 8.5 - Insoluble (%) < 5 - HEAVY METALS DETERMINATION SPECIFICATION Arsenic (As, mg/kg) < 1 Cadmium (Cd, mg/kg) < 1 Chrome (Cr, mg/kg) < 8 Iron (Fe, mg/kg) < 300 Manganese (Mn, mg/kg) < 10 Mercury(Hg, mg/kg) < 1 Nickel (Ni, mg/kg) < 8 Lead (Pb, mg/kg) < 1 Antimony (Sb, mg/kg) < 2 Selenium (Se, mg/kg) < 2 - Bromate ion (BrO3 , mg/kg) < 121 - Chlorate ion (ClO3 , g/kg) < 21.1 The present information is only to offer an orientation of the specific characteristics of the product delivered by Manuchar International. We recommend that buyers, when using our products, perform preliminary tests to determine that the characteristics of the product are suitable for their particular purposes. No authorization or guarantee is expressed or implied with respect to the data. Nothing in this publication constitutes a permission, insinuation or recommendation to implement inventions covered by any patent, nor as a recommendation for the use of any product or practice any procedure in violation of the law or government regulation; Therefore there should be no responsibility assigned to Manuchar International once the delivery has been made.