Full-Text (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Modeling Malaria Cases Associated with Environmental Risk Factors in Ethiopia Using Geographically Weighted Regression
MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING GEOGRAPHICALLY WEIGHTED REGRESSION Berhanu Berga Dadi i MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING THE GEOGRAPHICALLY WEIGHTED REGRESSION MODEL, 2015-2016 Dissertation supervised by Dr.Jorge Mateu Mahiques,PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain Ana Cristina Costa, PhD Professor, Nova Information Management School University of Nova Lisbon, Portugal Pablo Juan Verdoy, PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain March 2020 ii DECLARATION OF ORIGINALITY I declare that the work described in this document is my own and not from someone else. All the assistance I have received from other people is duly acknowledged, and all the sources (published or not published) referenced. This work has not been previously evaluated or submitted to the University of Jaume I Castellon, Spain, or elsewhere. Castellon, 30th Feburaury 2020 Berhanu Berga Dadi iii Acknowledgments Before and above anything, I want to thank our Lord Jesus Christ, Son of GOD, for his blessing and protection to all of us to live. I want to thank also all consortium of Erasmus Mundus Master's program in Geospatial Technologies for their financial and material support during all period of my study. Grateful acknowledgment expressed to Supervisors: Prof.Dr.Jorge Mateu Mahiques, Universitat Jaume I(UJI), Prof.Dr.Ana Cristina Costa, Universidade NOVA de Lisboa, and Prof.Dr.Pablo Juan Verdoy, Universitat Jaume I(UJI) for their immense support, outstanding guidance, encouragement and helpful comments throughout my thesis work. Finally, but not least, I would like to thank my lovely wife, Workababa Bekele, and beloved daughter Loise Berhanu and son Nethan Berhanu for their patience, inspiration, and understanding during the entire period of my study. -

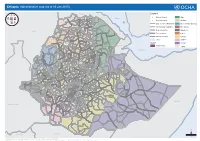
Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

Benishangul-Gumuz Region
Situation Analysis of Children and Women: Benishangul-Gumuz Region Situation Analysis of Children and Women: Benishangul-Gumuz Region ABSTRACT The Situation Analysis covers selected dimensions of child well-being in Benishangul-Gumuz Regional State. It builds on the national Situation Analysis of Children and Women in Ethiopia (2019) and on other existing research, with inputs from specialists in Government, UNICEF Ethiopia and other partners. It has an estimated population of approximately 1.1 million people, which constitutes 1.1% of the total Ethiopian population. The population is young: 13 per cent is under-five years of age and 44 per cent is under 18 years of age. Since 1999/00, Benishangul-Gumuz has experienced an impressive 28 percentage point decline in monetary poverty, but 27 per cent of the population are still poor; the second highest in the country after Tigray and higher than the national average of 24 per cent. SITUATION ANALYSIS OF CHILDREN AND WOMEN: BENISHANGUL-GUMUZ REGION 4 Food poverty continued a steep decline from 55 per cent in 1999/00 to 24 per cent in 2015/16; close to the national average of 25 per cent. In Benishangul-Gumuz, in 2014, only 1.1 per cent of rural households were in the PSNP compared to 11 per cent of households at the national level In 2011, the under-five mortality rate in Benishangul-Gumuz was the highest in Ethiopia (169 per 1,000 live births); this declined significantly, but is still very high: 96 deaths per 1,000 births, which is the second highest in the country after Afar. -

Cross Sectional Study on Prevalence of Bovine Trypanosomosis and Associated Risk Factors in Mao Komo Special Woreda, Benishahgul Gumuz, Western Ethiopia
Vol. 10(4), pp. 45-50, April 2018 DOI: 10.5897/JPVB2017.0311 Article Number: 03CF66F56701 Journal of Parasitology and ISSN 2141-2510 Copyright © 2018 Vector Biology Author(s) retain the copyright of this article http://www.academicjournals.org/JPVB Full Length Research Paper Cross sectional study on prevalence of bovine trypanosomosis and associated risk factors in Mao komo special woreda, benishahgul gumuz, Western Ethiopia Geremew Haile1* and Oda Gizaw2 1Department of Veterinary Medicine, Wollega University, P. O. Box 395, Nekemt, Ethiopia. 2Department of Animals Science, College of Agriculture, Mettu University, Bedelle, Ethiopia. Received 15 November, 2017; Accepted 26 February, 2018 Trypanosomosis is a chronic haemo-protozoal disease, which is a barrier to livestock and agricultural production. A cross-sectional study with the aim of determining prevalence rate of bovine trypanosomosis and risk factors was carried out from April 2016 to November 2017 in the Mao Komo district in the Benishangul-gumuz, Ethiopia. Buffy coat followed by thin blood smear technique were employed to identify the species of the trypanosomes. Out of total 384 cattle examined, 18 were infected with trypanosomes with the overall prevalence of 4.69%. High prevalence of Trypanosoma congolense was recorded in the area (65.7%) followed by Trypanosoma vivax and Trypanosoma brucei. The study revealed that there was statistically significant difference (p<0.05) of the prevalence in cattle with body condition. This is explained as there was higher prevalence of the diseases in cattle with poor body condition than medium and good body condition. Relatively higher prevalence was found in females and adult animals, and Fafafa peasant association but there was no significant (p>0.05) difference between the variables. -

Prevalence of Bovine Trypanosomosis and Tsetse Fly Density in Different
ary Scien in ce r te & e T V e Alembo, J Vet Sci Technol 2019, 10:4 f c h o n l Journal of Veterinary o a l n o r g u y o J Science & Technology ISSN: 2157-7579 Review Article Open Access Prevalence of Bovine Trypanosomosis and Tsetse Fly Density in Different Regions of Ethiopia: A Review Edget Abayneh Alembo* Department of Animal Sciences, College of Agricultural Science, Arba Minch University, Arba Minch, Ethiopia Abstract Ethiopia is known for its large and diverse livestock resource endowments and Bovine Trypanosomosis has long been recognized as a massive constraint on animal husbandry, livestock production and mixed farming in vast areas of rural sub-Saharan Africa. In Ethiopia, trypanosomosis is widespread in domestic livestock in the Western, South and Southwestern lowland regions and the associated river systems. The tsetse flies in Ethiopia are confined to the southern and western regions. Out of nine region of Ethiopia, five (Amhara area, Benshangul-Gumuzs, Gambella, Oromia and Southern Nations Nationalities and Peoples‟ Regional State) are infected with more than one species of tsetse flies and there are five species of tsetse flies in those mentioned regions. For this systematic review more than 60 published paper from 2000-2019 which were done in Amhara, Oromia, SNNPRs, Benshamgul Gumize and Gambella regions, respectively. According to this review the overall prevalence of bovine trypanosomosis in five regions of Ethiopia revealed that 8.6%, 9.3%, 11.2%, 10.6% and 18.1% in Amhara, Oromia, SNNPRs, Benshamgul Gumize and Gambella, respectively. Furthermore this review indicted that high infestation of tsetse fly in Oromia region by four species namely Glossina pallidipes, Glossina morsitans, Glossina fuscipes and Glossina tachinoide followed by G. -
Oromiya 4 Zone Code Wereda Code Town Code West
REGION - OROMIYA 4 ZONE CODE WEREDA CODE TOWN CODE WEST WELEGA 01 MENE SIBU 01 MENDI 1 NEJO 02 NEJO 1 WERE JIRU 2 GORI 3 GIMBI 03 HOMA 1 LALO ASABI 04 ENANGO 1 DENIGORO 2 KILTU KARA 05 KILTU KARA 1 BOJI DIRMEJ 06 BILA 2 AYIRA GULISO 07 GULISO 1 AYIRA 2 JARSO 08 GEBA DEFINO 1 KONDALA 09 GEBA DEFNO 1 BOJI CHEKORSA 10 CHEKORSA 1 BABO GAMBEL 11 DEBEKA 1 YUBDO 12 YUBDO 1 GENJI 13 GENJI 1 HARU 14 GUYU 1 NOLE KABA 15 BUBE 1 BEGI 16 BEGI 1 KOBER 2 GIMBI /TOWN/ 17 GIMBE 1 SEYO NOLE 18 DEBESO 1 EAST WELEGA 02 LIMU 01 GELILA 1 IBANTU 02 HINDE 1 GIDA KIREMU 03 GIDA AYANA 1 KIREMU 2 GUTEN 3 HORO LIMU 04 BONIYA BUSHE 05 BILO 1 WAYU TUKA 06 GEBA JIMATA 1 GUDEYA BILA 07 JERE 1 BILA 2 GOBU SEYO 08 ANO 1 OROMIYA (Cont'd) ZONE CODE WEREDA CODE TOWN CODE EAST WELEGA 02 SIBU SIRE 09 SIRE 1 DIGA 10 ARJO GUDETU 1 IFA 2 SASIGA 11 GALO 1 LEKA DULECHA 12 GETEMA 1 GUTO GIDA 13 DIGA 1 JIMA ARJO 14 ARJO 1 NUNU KUMBA 15 NUNU 1 WAMA HAGELO 16 NEKEMTE /TOWN/ 17 NEKEMTE 1 ILU ABA BORA 03 DARIMU 01 DUPA 1 ALGE SACHI 02 ALGE 1 SUPE 2 CHORA 03 KUMBABE 1 DEGA 04 DEGA 1 DABO HANA 05 KONE 1 GECHI 06 GECHI 1 BORECHA 07 YANFU 1 DEDESA 08 DENBI 1 YAYU 09 YAYU 1 METU ZURIYA 10 ALE 11 GORE 1 BURE 12 BURE 01 1 SIBU 2 NONO SELE 13 BIRBIRSA 1 OROMIYA (Cont'd) ZONE CODE WEREDA CODE TOWN CODE ILU ABA BORA 03 BICHO 14 BICHO 1 BILO NOPHA 15 NOPA 1 HURUMU 16 HURUMU 1 DIDU 17 LALO 1 MAKO 18 MAKO 1 HUKA /HALU/ 19 HUKA 01 1 METU TOWN 20 METU 1 BEDELE TOWN 21 BEDELE 1 BEDELE ZURIYA 22 - 1 CHEWAKA 23 ILU HARERE 1 DORENI 24 JIMA 04 LIMU SEKA 01 ANTAGO 1 LIMU KOSA 02 LIMU GENET 1 AMBUYE 2 BABU -

Project Implementation Manual Final Draft
THE NATIONAL REGIONAL GOVERNMENT OF OROMIA OROMIA ENVIRONMENT, FOREST AND CLIMATE CHNAGE AUTHORITY Oromia National Regional State Forested Landscape Program (Project ID P156475) Project Implementation Manual Final Draft (2017-2022) Addis Ababa, Ethiopia April 25, 2017 i Abbreviations and Acronyms A/R Afforestation/Reforestation AD Activity Data AF Additional Finance AGP Agricultural Growth Program AWP&B Annual Work Plan and Budget BioCF BioCarbon Fund BMERP Bale Mountains Eco-region REDD+ Project BoANR Bureau of Agriculture and Natural Resource BoFEC Bureaus of Finance and Economic Cooperation BoLFD Bureau of Livetsock and Fishery Development BoRLAU Bureau of Rural Land Adminsitration and Use BoWME Bureau of Water, Minerals and Energy BSM Benefit Sharing Mechanism CBO Community Based Organization CPO Cooperative Promotion Office CQS Selection Based Consultants’ Qualifications CRGE Climate Resilient Green Economy DA Development Agent EIO Ethiopian Institute of Ombudsman EMA Ethiopian Mapping Agency ER Emissions Reductions ERPA Emissions Reductions Purchase Agreement ESMF Environmental and Social Management Framework FA Force Account FAO Food and Agriculture Organization of the United Nations FREL Forest Reference Emission Level FRL Forest Reference Level GHG Greenhouse GIS Geographic Information System GoE Government of Ethiopia GRM Grievance Redress Mechanism ii GTP Growth and Transformation Plan IBRD International Bank of Reconstruction and Development ICB International Competitive Bidding ICS Improved Cook Stoves IDA International Development -
Ethiopia: Administrative Map (As of 05 Jan 2015)
Ethiopia: Administrative map (as of 05 Jan 2015) ERITREA Legend Ahferom Erob ^! Tahtay Adiyabo National Capital Gulomekeda Afar Laelay Adiyabo Mereb Leke Red Sea Dalul Ganta Afeshum P! Adwa SaesEiea Tsatedranemba Regional Capital Amhara North WesternTahtay KoraroLaelay Maychew Kafta Humera Tahtay Maychew Werei Leke Hawzen Asgede Tsimbila Central Koneba TIGRAY Medebay Zana Naeder Adet Berahile Western Atsbi Wenberta Undetermined boundary Beneshangul Gumuz Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele P! Zone 2 International boundary Dire Dawa Tsegede Tselemt Enderta Tanqua Abergele Afdera Addi Arekay Ab Ala Tsegede Beyeda Mirab Armacho Afdera Region boundary Gambela Debark Saharti Samre Erebti Hintalo Wejirat SUDAN Abergele Tach Armacho Dabat Janamora Megale Bidu Alaje Sahla Southern Zone boundary Hareri Ziquala Raya Azebo Metema Lay Armacho Wegera Wag Himra Endamehoni North Gonder Sekota Teru Chilga Woreda boundary Oromia Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Zone 4 Elidar Gaz Gibla Lake SNNPR Quara Takusa Ebenat Gulina Libo Kemkem Bugna Awra Kobo Tana Gidan Region Lasta (Ayna) AFAR Somali Alfa Ewa Fogera Farta North Wollo Semera Lay Gayint Meket Guba Lafto P! Dubti Zone 1 DJIBOUTI Addis Ababa Jawi Semen Achefer South Gonder Tigray East Esite Chifra Bahir Dar Wadla Habru Aysaita AMHARA P! Dera Tach Gayint Delanta Guba Bahirdar Zuria Ambasel Debub Achefer Dawunt Worebabu Dangura Gulf of Aden Mecha West Esite Simada Thehulederie Adaa'r Mile Pawe Special Afambo Dangila Kutaber Yilmana -

Prevalence of Bovine Trypanosomosis, Tsetse Density and Farmers
ary Scien in ce r te & e T V e f c h o Journal of Veterinary Science & n n l Kassaye and Tsegaye, J Veterinar Sci Technol 2016, 7:2 o o a a l l n n o o r r g g u u DOI: 10.4172/2157-7579.1000295 y y o o J J Technology ISSN: 2157-7579 Research Article Open Access Prevalence of Bovine Trypanosomosis, Tsetse Density and Farmers Perceptions on the Impact of Control Program in Kellem Wollega Zone, Western Oromia, Ethiopia Bedaso Kebede Kassaye1* and Dereje Tsegaye2 1Veterinary Drug and Animal Feed Administration and Control Authority, Ministry of Agriculture, Addis Ababa, Ethiopia 2North Shoa/Selale Livestock development and Health office, Ethiopia *Corresponding author: Bedaso Kebede Kassaye, Veterinary Drug and Animal Feed Administration and Control Authority, Ministry of Agriculture, Addis Ababa, Ethiopia, Tel: 0913136824; E-mail: [email protected] Rec date: Nov 10, 2015; Acc date: Jan 08, 2016; Pub date: Jan 11, 2016 Copyright: © 2016 Kassaye BK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract A study was conducted from November 2011 to April 2012 Dale Wabera and Dale Sadi Districts of Kellem Wollega Zone of western Oromia. The objectives of the study were to assess the owners' perception on the impact of Trypanosomosis and its control, determine the prevalence of bovine Trypanosomosis and to assess the distribution and apparent densities of vectors of the Trypanosomosis. -
Urban Water Supply Universal Access Plan.Pdf
Federal Democratic Republic of Ethiopia Ministry of Water and Energy PART III Urban Water Supply Universal Access Plan (UWSPUAP) 2011-2015 December 2011 Addis Ababa 1 URBAN WATER SUPPLY UAP Table of Contents Executive Summary .............................................................................................................................................. 1 1. Introduction ................................................................................................................................................... 2 2. Background ................................................................................................................................................... 2 3. The Urban UAP model and Assumptions ..................................................................................................... 4 4. Sector policy and Strategy ............................................................................................................................. 7 5. Cross cutting Issues ..................................................................................................................................... 10 6. Physical Plan ............................................................................................................................................... 10 7. Financial Plan .............................................................................................................................................. 12 7.1. Financial plan for Project Implementation ......................................................................................... -

National Wash Program Selected Woredas and Small Towns
National WaSH Program Selected Woredas and Small Towns 1. Oromiya Region No Oromiya Woredas No Oromiya Woredas 41 Dugda 83 Ana Sora No Oromiya Woredas 42 Liban Chukala 84 Seba Boru 1 Digalu Tijo 43 Lume 85 Uraga 2 Gololcha 44 Bora 86 Odo Shakkiso 3 Jeju 45 Gudeya Bila 87 Liban 4 Limu Bilbilo 46 Guto Gida 88 Fentale 5 Tiyo 47 Sasiga 89 Babile 6 Zuway Dugda 48 Sibu Sire 90 Chinaksen 7 Adaba 49 Wayu Tuka 91 Qumbi 8 Gedeb Asasa 50 Seyo Nole 92 Guba Korcha 9 Agarfa 51 Nedjo 93 Chiro 10 Dawe Serer 52 Haru 94 Chewaka 11 Dinsho 53 Dale Sedi 95 Hurum 12 Gindhir 54 Dale Wabera 96 Darimu 13 Goro 55 Gawo Kebe 97 Dedo 14 Rayitu 56 Lalo Kile 98 Sokoru 15 Sinana 57 Seyo 99 Seka Chokorsa 16 Bedeno 58 Yubdo 100 Limu Seka 17 Gorogutu 59 Ayira 101 Ilu 18 Girawa 60 Guliso 102 Sodo Dachi 19 Kurfa Chale 61 Sude 103 Weliso 20 Kombolcha 62 Doddota 104 Ameya 21 Haromaya 63 Inkolo Wabe 105 Seden Soddo 22 Kersa 64 Robe 106 Nonno 23 Fedis 65 Lege Hidha 107 Dera 24 Boke 66 Sewena 108 Girar Jarso 25 Darulabu 67 G/Dhamole 109 Yaya Gulele 26 Doba 68 Siraro 110 Kuyu 27 Oda Bultum 69 Arsi Negele 111 Wuchale 28 Tulo 70 Kofele 112 Sululta 29 Hawi Gudina 71 Shashemene 113 Mulo 30 Burqa Dimtu 72 Kokosa 114 Sebeta Hawas 31 Becho 73 Akaki 115 Wolmera 32 Bedele 74 Teltale 116 Berek 33 Bilo Nopha 75 Dhas 117 Dendi 34 Didu 76 Yabello 118 Meta Robi 35 Metu 77 Arero 119 Abuna Ginde Beret 36 Yayu 78 Bule Hora 120 Ambo 37 Dorani 79 Gelana 121 Ilu Gelan 38 Adea 80 Moyale 122 Jibat 39 Adama 81 Hambela Wamana 123 Mida Kegn 40 AT/Jido Kombolcha 82 Kercha 124 Toke Kutaye National WaSH