Annual Report 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A. HM, B. Spa@Al Naviga@On 2. Hippocampus
Outline 1. What does Hippocampus do? A. HM, B. Spaal navigaon 2. Hippocampus anatomy and connec;vity CA1, CA3 diagram etc. Connec;vity to other regions. 3. Place fields in Hippocampus – show examples + the O'Keefe effect 4. Plas;city of place fields + NMDA blocker + Morris water maze 5. Replay of sequences 6. Entorhinal grid cells What does the Hippocampus do? 1. Memory 2. Spaal representaon Henry Gustav Molaison (February 26, 1926 – December 2, 2008), beger known as HM “AYer operaon this young man could no longer recognize the hospital staff nor find his way to the bathroom, and he seemed to recall nothing of the day-to-day events of his hospital life. There was also a par;al retrograde amnesia, inasmuch as he did not remember the death of a favorite uncle three years previously, nor anything of the period in the hospital, yet could recall some trivial events that had occurred just before his admission to the hospital. His early memories were apparently vivid and intact. This paent’s memory defect has persisted without improvement to the present ;me, and numerous illustraons of its severity could be given. Ten months ago the family moved from their old house to a new one a few blocks away on the same street; he s;ll has not learned the new address, though remembering the old one perfectly, nor can he be trusted to find his way home alone.” “Moreover, he does not know where objects in con;nual use are kept; for example, his mother s;ll has to tell him where to find the lawn mower, even though he may have been using it only the day before. -

Press Release Below to Your Local Media
Please forward the press release below to your local media. Thank you. Press Release: USA Student Wins World Neuroscience Competition August, 2017; Washington, DC, USA Future neuroscientists from 25 countries around the world met in Washington, DC this week to compete in the nineteenth World Brain Bee Championship. The Brain Bee is a neuroscience competition for young students, 13 to 19 years of age. It was hosted by the American Psychological Association. The International Brain Bee President and Founder is Dr. Norbert Myslinski ([email protected]) of The University of Maryland Dental School, Department of Neural and Pain Sciences. Its purpose is to motivate young men and women to study the brain, and to inspire them to enter careers in the basic and clinical neurosciences. We need bright young men and women to help treat and find cures for the 1000 neurological and psychological disorders around the world. The Brain Bee "Builds Better Brains to Fight Brain Disorders.” The 2017 World Brain Bee Champion is Sojas Wagle, a sophomore from Har-Ber High School in Arkansas, USA. Legend: Sojas Wagle Sojas has a breath taking history of accomplishments. He is the captain of his school’s Quiz Bowl Team, and was state MVP for the last two years. He placed third in the National Geographic Bee in 2015. Last year, he was chosen for “Who Wants to be a Millionaire” Whiz Kids Edition. By the end of the game show, he had won $250,000, and later donated some of his winnings to his school district and a children’s hospital. -

Retrohippocampal Cortical Neurons During Hippocampal Sharp Waves in the Behaving Rat
The Journal of Neuroscience, October 1994, 74(10): 6160-6170 Selective Activation of Deep Layer (V-VI) Retrohippocampal Cortical Neurons during Hippocampal Sharp Waves in the Behaving Rat J. J. Chrobak and G. Buzsaki Center for Molecular and Behavioral Neuroscience, Rutgers, The State University of New Jersey, Newark, New Jersey 07102 The coordinated activity of hippocampal neurons is reflected feet on their postsynaptic neocortical targets and may rep- by macroscopic patterns, theta and sharp waves (SPW), ev- resent a physiological mechanism for memory trace transfer ident in extracellular field recordings. The importance of these from the hippocampus to the neocortex. patterns is underscored by the ordered relation of specific [Key words: hippocampus, entorhinal cortex, theta, sharp neuronal populations to each pattern as well as the relation waves, oscillations, memory, temporal lobe epilepsy, Ab- of each pattern to distinct behavioral states. During awake heimer’s disease] immobility, consummatory behavior, and slow wave sleep, CA3 and CA1 neurons participate in organized population Retrohippocampal structures [entorhinal cortex (EC), parasub- bursts during SPW. In contrast, during theta-associated ex- iculum, presubiculum, and subiculum] process and transmit ploratory activity, the majority of principle cells are silent. information between the neocortex and the hippocampus. The Considerably less is known about the discharge properties electrophysiology of thesestructures has received scant attention of retrohippocampal neurons during theta, and particularly despite their importance as a substrate for memory (Amaral, during SPW. These retrohippocampal neurons (entorhinal 1987; Zola-Morgan et al., 1989; Squire, 1992)and asfocal point cortical, parasubicular, presubicular, and subicular) process for the pathophysiology of dementia (Hyman et al., 1984; Van and transmit information between the neocortex and the hip- Hoesenet al., 1991) and temporal lobe epilepsy (Rutecki et al., pocampus. -

Creating a Web-Based 3D Interactive Resource to Teach the Anatomy of the Human Hippocampus
Morphology of Memory: Creating a web-based 3D interactive resource to teach the anatomy of the human hippocampus by Alisa M. Brandt A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Arts Baltimore, Maryland March, 2019 © 2019 Alisa Brandt All Rights Reserved ABSTRACT The hippocampus is a critical region of the brain involved in memory and learning. It has been widely researched in animals and humans due to its role in consolidating new experiences into long-term declarative memories and its vulnerability in neurodegenerative diseases. The hippocampus is a complex, curved structure containing many interconnected regions that consist of distinct cell types. Despite the importance of understanding the normal state of hippocampal anatomy for studying its functions and the disease processes that affect it, didactic educational resources are severely limited. The literature on the hippocampus is expansive and detailed, but a communication gap exists between researchers presenting hippocampal data and those seeking to improve their understanding of this part of the brain. The hippocampus is typically viewed in a two-dimensional fashion; students and scientists have diffculty visualizing its three-dimensional anatomy and its structural relationships in space. To improve understanding of the hippocampus, an interactive, web-based educational resource was created containing a pre-rendered 3D animation and manipulable 3D models of hippocampal regions. Segmentations of magnetic resonance imaging data were modifed and sculpted to build idealized anatomical models suitable for teaching purposes. These models were animated in combination with illustrations and narration to introduce the viewer to the subject, and the completed animation was uploaded online and embedded into the interactive. -

Institute of Cognitive Science Newsletter - Winter 2016
Institute of Cognitive Science Newsletter - Winter 2016 From the Director of the Institute Dear Colleagues, As my first semester as Director draws to a close, I want to thank all of you for your kindness, energy, and support during this start-up period. I continue to be amazed by the depth and breadth of research being conducted by our faculty, fellows, and students. Thanks to your hard work, and the leadership of our former Director Marie Banich, the Institute of Cognitive Science has laid the groundwork for transformative future growth through developing significant human, technological, and capital infrastructure over the past decade. We have a suite of talented and creative interdisciplinary tenure track and research faculty, a broad range of faculty fellows from seven participating units, and a productive and well-functioning staff (human). Our research has advanced science in learning, language, and higher order cognition, with a core emphasis on developing and using innovative computational approaches and neuroimaging to study, model, and support human cognition (technological). This research is made possible through our ongoing operation of a state-of-the-art neuroimaging facility and the CINC laboratory facility (capital). As you can see later in this newsletter, our research and education programs are strong and vital: we are steadily receiving new grants, expanding our faculty and fellows, and producing outstanding graduates. However, our challenge in the years ahead is to devise new ways of working together so that we can build on our infrastructure to take our contributions to science and society to a new level. I firmly believe that the Institute is uniquely poised to tackle some of society’s most pressing challenges: understanding brain health and wellness, developing personalized therapies and interventions, personalizing and deepening learning, and optimizing complex cognitive processes to improve performance and outcomes. -

2018 IBB Prgbk V1000
Program Berlin,Germany July 5th-9th 2018 Welcome to Germany! It is a true privilege to welcome you to the 20th International Brain Bee (IBB) World Champi- onship in Berlin, Germany, organised in conjunction with the 11th Forum of the Federation of European Neuroscience Societies (FENS). We would like to congratulate all participants on reaching the IBB World Championship. You are the best in your countries and this represents an exceptional achievement! We look forward to a friendly, inspiring but also challenging competition this year in Berlin! The 2018 IBB will allow you to test and expand your neuroscience knowledge. During the neu- roanatomy exam, you will have a chance to analyze real brain specimens and then for an hour you will become a neurologist who will diagnose patients suffering from neurological disorders. Your knowledge and speed will be challenged in a written exam and in the live podium session where you will be intellectually engaged by a panel of world-renowned neuroscientists. Like the real scientific community, the IBB is not only about knowledge but also about meeting new people that share passion for neuroscience. During your stay in Berlin, you will be encouraged to get to know fellow national champions and build connections and friendships that have the potential to last a lifetime. Furthermore, in the IBB you will meet experienced neuroscientists who will serve as role models for your future career. Therefore, we recommend reaching out to and socializing as much as possible with them to make the most out of the 2018 IBB experience! All attendees will also be invited to join the International Youth Neuroscience Association (IYNA), which was created to connect the IBB alumni and promote neurosciences in schools across the world. -

Traumatic Early Life Stress in the Developing Hippocampus: a Meta-Analysis of MRI Studies
Walden University ScholarWorks Walden Dissertations and Doctoral Studies Walden Dissertations and Doctoral Studies Collection 2020 Traumatic Early Life Stress in the Developing Hippocampus: A Meta-Analysis of MRI Studies Sharon Johnson Walden University Follow this and additional works at: https://scholarworks.waldenu.edu/dissertations Part of the Psychology Commons This Dissertation is brought to you for free and open access by the Walden Dissertations and Doctoral Studies Collection at ScholarWorks. It has been accepted for inclusion in Walden Dissertations and Doctoral Studies by an authorized administrator of ScholarWorks. For more information, please contact [email protected]. Walden University College of Social and Behavioral Sciences This is to certify that the doctoral dissertation by Sharon Lee Johnson has been found to be complete and satisfactory in all respects, and that any and all revisions required by the review committee have been made. Review Committee Dr. Scott Wowra, Committee Chairperson, Psychology Faculty Dr. Patricia Costello, Committee Member, Psychology Faculty Dr. Kimberley Cox, University Reviewer, Psychology Faculty Chief Academic Officer and Provost Sue Subocz, Ph.D. Walden University 2020 Abstract Traumatic Early Life Stress in the Developing Hippocampus: A Meta-Analysis of MRI Studies by Sharon Lee Johnson MPhil, Walden University, 2019 M.Ed., Youngstown State University, 1994 BA, Kent State University, 1979 Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Health Psychology Walden University August 2020 Abstract Advancements in neuroimaging techniques afford researchers the opportunity to examine the actual brains of living persons, which exponentially contributes to new insights regarding brain and behavior phenomena. However, empirical studies investigating stress and the hippocampus attend primarily to adult populations - less on children and adolescents. -

Abstracts PDF Theme H
When citing an abstract from the 2017 annual meeting please use the format below. [Authors]. [Abstract Title]. Program No. XXX.XX. 2017 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2017. Online. 2017 Copyright by the Society for Neuroscience all rights reserved. Permission to republish any abstract or part of any abstract in any form must be obtained in writing by SfN office prior to publication. Theme J Poster 021. History of Neuroscience Location: Halls A-C Time: Saturday, November 11, 2017, 1:00 PM - 5:00 PM Program#/Poster#: 021.01SA/VV14 Topic: J.01. History of Neuroscience Title: Neuroplasticity: Past, present and future Authors: *J. E. KOCH; Univ. WI Oshkosh, Oshkosh, WI Abstract: The concept and process of neuroplasticity and neuroplastic adaptations to differing environmental and sensory stimuli is accepted today as a fundamental capacity of the brain, however, the idea that the brain is a malleable integrated system is historically relatively recent. The focus of numerous 19th century scientists, including Gall, Flourens, Broca, and Sherrington resulted in a widespread belief among neuroscientists in both localization of function and the “fixed nature” of functional neuroanatomy. Further work by 20th century neuroscientists, such as Penfield’s brain mapping, solidified support for this approach. Challenges to this interpretation of the static nature of the brain started to appear around the middle of the 20th century, eventually resulting in a paradigm shift to the perspective that the brain is continually responsive and changing over the lifespan. This presentation covers the history of neuroscience’s change in perspective from “static to plastic” brains, describing pioneers and their research which resulted in the current level of knowledge and acceptance about neuroplasticity. -

IBB 2013 Program Online Ver
Welcome! On behalf of the 2013 Organizing Committee, it is a true privilege to introduce the 15th International Brain Bee Championship at the The 2013 International Brain Bee World Congress of Neurology in Vienna, Austria. Congratulations to - Organizing Committee - all student participants on their exceptional achievements, and we look forward to an enriching and inspiring competition and event. Norbert Myslinski, University of Maryland, USA Judith Shedden, McMaster University, Canada We celebrate a historic step this year in the development of the Linda J. Richards, The University of Queensland, Australia program. For the first time in the event's history, all six continents are represented, with a record-breaking number of student Jonathan Dostrovsky, University of Toronto, Canada participants. Further propelling the special event into the next Andrii Cherninskyi, Kiev National Taras Shevchenko chapter of its growth, the program encompasses four days of University, Ukraine engaging with our host conference and local partner institutions, the Branavan Manoranjan, McMaster University, Canada application of trilingual competition material for participants from Francis A. Fakoya, St. George’s University, Grenada non-English speaking regions, group explorations of the remarkable Meggie Mwoka, Ubongo Kenya capital city of Vienna, the establishment of the Patient Partner Nchafatso Gikenyi Obonyo, University of Nairobi, Kenya Program, and the support of lasting community among participants Cristian Gurzu, Romania with an online Alumni Association. We are delighted to provide the Polycarp Nwoha, Obafemi Awolowo University, Nigeria framework for an experience of a lifetime and hope each participant Julianne McCall, Heidelberg University, Germany is charged with the motivation to contribute to advancing science and humanity. -

Vol.27 Num(11)
www.neuroscience.utoronto.ca o UNIVERSITY OF TORONTO NEUROSCIENCE PROGRAM 1 NEUROSCIENCE NEWSLETTER University of Toronto Neuroscience Program Newsletter Summer 2011 | Volume 27 | Number 11 UTNP Administration UPCOMING DISTINGUISHED LECTURESHIP SERIES 2011-2012 TH Michael G. Fehlings WEDNESDAY, OCTOBER 12 , 2011 Director, UTNP Speaker | Dr. Gail Mandel, Senior Scientist at the Vollum Institute and Professor in the Department of Biochemistry and Molecular Biology in the School of Medicine at Oregon Health Sciences University. Topic | The rise and fall of REST: Creating a nervous system David R. Hampson TH WEDNESDAY, OCTOBER 26 , 2011 Director, CPIN Speaker | Dr. Nicholas P. Franks, Professor of Biophysics and Anaesthetics, Head of Division of Cell and Molecular Biology, Head of Biophysics Section, Blackett Laboratory, Imperial College London UTNP Host | Dr. Richard L. Horner, PhD Topic | TBA Alexander A. Velumian THURSDAY, NOVEMBER 3RD, 2011 Planning & Operations Speaker | Dr. Richard Morris, Centre for Cognitive and Neural Systems, University of Edinburgh, UK Topic | TBA Tallie Rabin-Claassen Interim Program Coordinator TH MONDAY, DECEMBER 12 , 2011 Speaker | Dr. Michael E. Goldberg UTNP Administrative Office | MP 11-315, David Mahoney Professor of Brain and Behavior, Director of The Toronto Western Hospital Mahoney Center at Columbia University (New York) 399 Bathurst St., Toronto, Ontario M5T 2S8 Topic | TBA Collaborative Program in Neuroscience | Room 904, Leslie Dan Faculty of Pharmacy, 144 College St., Toronto Ontario M5S 3M2 TH TUESDAY, APRIL 24 , 2012 University of Toronto Speaker | Dr. Keith Cicerone Director of Neuropsychology, JFK Johnson Rehabilitation Institute & Email | [email protected] New Jersey Neuroscience Institute, JFK Medical Center, New Jersey Phone | 416 978 8761 UTNP Host | Dr. -
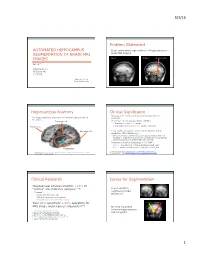
5/3/16 1 Automated Hippocampus Segmentation of Brain
5/3/16 Problem Statement AUTOMATED HIPPOCAMPUS • Goal: automated segmentation of hippocampus in SEGMENTATION OF BRAIN MRI brain MRI images Input Output hippocampus IMAGES Group 7 Yingchuan Hu Chunyan Wu Li Zhong May 1st, 2014 Cornell University Hippocampus Anatomy Clinical Significance • Epilepsy is the most common serious brain disorder The hippocampus is located in the medial temporal lobe of worldwide. the brain. 1 Temporal lobe • Prevalence of epilepsy worldwide (WHO ) • 7 sufferers in every 1,000 people • 3 new sufferers in every 10,000 people each year Frontal lobe Occipital lobe • People with epilepsy are at increased risks for status epilepticus (life-threatening) • One continuous, unremitting seizure lasting longer than five minutes or recurrent seizures without regaining consciousness between seizures for greater than five minutes. • Prevalence of status epilepticus in US (NIH2) • 195,000 new patients of status epilepticus each year • 42,000 deaths caused by status epilepticus each year 1. World Health Organization http://www.who.int/mental_health/neurology/epilepsy/en/ *Modified from a scan of a plate of “Posterior and inferior cornua of left lateral ventricle exposed 2. National institute of Health http://www.ninds.nih.gov/disorders/epilepsy/detail_epilepsy.htm from the side” in Gary’s Anatomy Clinical Research Issues for Segmentation • Hippocampal volume reduction >10% of “normal” size indicates epilepsy.[1-4] • Low contrast to neighboring brain • “normal”: structures • People with the same age • Bilateral hippocampus comparison • Personal changes in more than 1 year • Over 90% sensitivity + 98% specificity for amgydala hippocampus [5-7] MRI image measurement diagnosis. • No clear boundary between hippocampus 1. Cook MJ, et al. -

Press Release Below to Your Local Media
Please forward the press release below to your local media. Thank you. Press Release: Polish Student Wins World Neuroscience Competition July, 2018; Berlin, Germany Future neuroscientists from 25 countries around the world met in Berlin, Germany this week to compete in the Twentieth Anniversary International Brain Bee (IBB) Championship. The 2018 IBB Champion is Piotr Oleksy from Poland. First: Piotr Oleksy Second: Giovanni De Gannes Third: Huai-Ying Huang The IBB is a neuroscience competition for young students between 13 to 19 years old. Its purpose is to inspire them to learn about the human brain, apply neuroscience to their daily lives, and motivate them to enter careers in the basic and clinical neurosciences. We need bright young men and women to help treat and find cures for the neurological and psychological disorders affecting millions of people around the world. Piotr Oleksy, this year s IBB Champion, is 18 years old and from the Liceum Ogolnoksztalcace High School in Krakow. He is interested in studying neurotransmitters and their receptors because he believes molecular’ neurology is the basis for finding cures for many disorders, such as schizophrenia and depression. His dream is to go to medical school. He also loves good music like Mahler, Glass, and Rachmaninoff, and great works of art from the Art Nouveau period. Second Place went to 14 year-old Giovanni De Gannes of Grenada Boys’ Secondary School in Grenada, West Indies. Giovanni appreciates hard work, team cooperation and living a balanced life. He also enjoys developing software, swimming competitively, and playing musical instruments. Third Place went to 17 year-old Huai-Ying Huang of Sir Frederick Banting Secondary School in London, Ontario, Canada.