Were Mammals Originally Venomous?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Miocene Mammal Reveals a Mesozoic Ghost Lineage on Insular New Zealand, Southwest Pacific
Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific Trevor H. Worthy*†, Alan J. D. Tennyson‡, Michael Archer§, Anne M. Musser¶, Suzanne J. Hand§, Craig Jonesʈ, Barry J. Douglas**, James A. McNamara††, and Robin M. D. Beck§ *School of Earth and Environmental Sciences, Darling Building DP 418, Adelaide University, North Terrace, Adelaide 5005, South Australia, Australia; ‡Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington 6015, New Zealand; §School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia; ¶Australian Museum, 6-8 College Street, Sydney, New South Wales 2010, Australia; ʈInstitute of Geological and Nuclear Sciences, P.O. Box 30368, Lower Hutt 5040, New Zealand; **Douglas Geological Consultants, 14 Jubilee Street, Dunedin 9011, New Zealand; and ††South Australian Museum, Adelaide, South Australia 5000, Australia Edited by James P. Kennett, University of California, Santa Barbara, CA, and approved October 11, 2006 (sent for review July 8, 2006) New Zealand (NZ) has long been upheld as the archetypical Ma) dinosaur material (13) and isolated moa bones from marine example of a land where the biota evolved without nonvolant sediments up to 2.5 Ma (1, 14), the terrestrial record older than terrestrial mammals. Their absence before human arrival is mys- 1 Ma is extremely limited. Until now, there has been no direct terious, because NZ was still attached to East Antarctica in the Early evidence for the pre-Pleistocene presence in NZ of any of its Cretaceous when a variety of terrestrial mammals occupied the endemic vertebrate lineages, particularly any group of terrestrial adjacent Australian portion of Gondwana. -

Recent Advances in Studies on Mesozoic and Paleogene Mammals in China
Vol.24 No.2 2010 Paleomammalogy Recent Advances in Studies on Mesozoic and Paleogene Mammals in China WANG Yuanqing* and NI Xijun Institute of Vertebrate Paleontology and Paleoanthropology, CAS, Beijing 10004, China ike in other fields of paleontology, research in from the articular of the lower jaw and the quadrate of the paleomammalogy mainly falls into two aspects. cranium following the process of reduction and detachment LOne is related to the biological nature of fossil from the reptilian mandible, are new elements of the bony mammals, such as their systematics, origin, evolution, chain in the mammalian middle ear. The appearance of phylogenetic relationships, transformation of key features mammalian middle ear allows mammals to hear the sound and paleobiogeography, and the other is related to the of higher frequencies than reptiles do. Generally speaking, geological context, involving biostratigraphy, biochronology, a widely accepted hypothesis is that mammals originated faunal turnover and its relations to the global and regional from a certain extinct reptilian group. The formation and environmental changes. In the last decade, a number of development of the definitive mammalian middle ear well-preserved mammalian specimens were collected from (DMME) has thus become one of the key issues in the study different localities around the country. Such discoveries have of mammalian evolution and has drawn the attention from provided significant information for understanding the early many researchers for many years. evolution of mammals and reconstructing the phylogeny of Developmental biological studies have proven the early mammals. function of the Meckel’s cartilage and its relationship to Studies of Chinese Mesozoic mammals achieved the ear ossicles during the development of mammalian remarkable progress in the past several years. -

Mammal Evolution
Mammal Evolution Geology 331 Paleontology Triassic synapsid reptiles: Therapsids or mammal-like reptiles. Note the sprawling posture. Mammal with Upright Posture From Synapsids to Mammals, a well documented transition series Carl Buell Prothero, 2007 Synapsid Teeth, less specialized Mammal Teeth, more specialized Prothero, 2007 Yanoconodon, Lower Cretaceous of China Yanoconodon, Lower Cretaceous of China, retains ear bones attached to the inside lower jaw Morganucodon Yanoconodon = articular of = quadrate of Human Ear Bones, or lower reptile upper reptile Auditory Ossicles jaw jaw Cochlea Mammals have a bony secondary palate Primary Palate Reptiles have a soft Secondary Palate secondary palate Reduction of digit bones from Hand and Foot of Permian Synapsid 2-3-4-5-3 in synapsid Seymouria ancestors to 2-3-3-3-3 in mammals Human Hand and Foot Class Mammalia - Late Triassic to Recent Superorder Tricodonta - Late Triassic to Late Cretaceous Superorder Multituberculata - Late Jurassic to Early Oligocene Superorder Monotremata - Early Cretaceous to Recent Superorder Metatheria (Marsupials) - Late Cretaceous to Recent Superorder Eutheria (Placentals) - Late Cretaceous to Recent Evolution of Mammalian Superorders Multituberculates Metatheria Eutheria (Marsupials) (Placentals) Tricodonts Monotremes . Live Birth Extinct: . .. Mammary Glands? Mammals in the Age of Dinosaurs – a nocturnal life style Hadrocodium, a lower Jurassic mammal with a “large” brain (6 mm brain case in an 8 mm skull) Were larger brains adaptive for a greater sense of smell? Big Brains and Early Mammals July 14, 2011 The Academic Minute http://www.insidehighered.com/audio/academic_pulse/big_brains_and_early_mammals Lower Cretaceous mammal from China Jawbones of a Cretaceous marsupial from Mongolia Mammal fossil from the Cretaceous of Mongolia Reconstructed Cretaceous Mammal Early Cretaceous mammal ate small dinosaurs Repenomamus robustus fed on psittacosaurs. -

Mesozoic: the Dark Age for Mammals!
Ed’s Simplified History of the Mammals Note progression from Pelycosaurs (1) to Therapsids and Cynodonts (2) in Triassic. Stem mammals appeared in Late Triassic and Early Jurassic (3). Relationships among the Middle Jurassic forms (4) are controversial (see handout). Therian clade, identified by the tribosphenic molar (5), emerged at the end of the Jurassic, Early Cretaceous. A slightly more detailed version… in case you like something that looks more slick From Pough et al. 2009. Vertebrate Life, 8th ed. Pelycosaurs Dominated the late Permian, gave rise to therapsids Therapsids Rapid radiation in late Permian, around 270 MYA Still “mammal-like reptiles” The mass extinction at the end of the Permian was the greatest loss of diversity ever with >80% of all known genera and about 90% of all species going extinct, both terrestrial and marine. Cynodonts Late Permian to mid Triassic Last remaining group of therapsids, survived mass extinction at the end of the Permian. Persisted well Only 1 lineage of into Triassic and developed cynodonts survived many features associated through the late Triassic, with mammals. and this group became ancestors of mammals. Mesozoic: the Dark Age for Mammals! multituberculate Morganucodon, one of the earliest mammals (What else was happening in the Late Triassic and Jurassic Hadrocodium that may have contributed to mammals becoming small and Most were very small with nocturnal?) conservative morphology ...but new fossil finds indicate more diversity than we thought Repenomanus Still, largest known mammal during Mesozic Most were shrew to is no larger than a mouse sized, for 125 woodchuck million years! Some Mesozoic events and mammals you should know 1. -
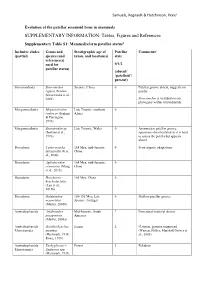
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

FROM the EARLY CRETACEOUS (BARREMIAN) WESSEX FORMATION OFTHEISLEOFWIGHT,SOUTHERNBRITAIN by STEVEN C
[Palaeontology, Vol. 49, Part 4, 2006, pp. 889–897] A GOBICONODONTID (MAMMALIA, EUTRICONODONTA) FROM THE EARLY CRETACEOUS (BARREMIAN) WESSEX FORMATION OFTHEISLEOFWIGHT,SOUTHERNBRITAIN by STEVEN C. SWEETMAN School of Earth and Environmental Sciences, University of Portsmouth, Burnaby Building, Burnaby Road, Portsmouth PO1 3QL, UK; e-mail: [email protected] Typescript received 16 June 2004; accepted in revised form 20 June 2005 Abstract: Bulk screening of Early Cretaceous (Barremian) gobiconodontid mammal in Early Cretaceous deposits of strata of the Wessex Formation, exposed in sections on Britain sheds further light on the palaeogeographical distri- the south-west and south-east coasts of the Isle of Wight, bution of an apparently successful clade of Early Creta- southern England, has resulted in the recovery of mammal ceous mammals and together with the occurrence of a remains, the first to be obtained from Wealden Group gobiconodontid in the earliest Cretaceous of North Africa strata since the early 1970s. The fauna comprises at least calls into question recent hypotheses concerning the area six taxa represented by isolated teeth and in addition, in of origin of the Gobiconodontidae and mechanisms of dis- the case of an as yet undescribed spalacotheriid, a partial persal therefrom. dentary. One of the teeth, a distal premolar, is of unique tricuspid, single-rooted morphology and represents the first Key words: Britain, Cretaceous, Gobiconodontidae, Mam- British record of the Gobiconodontidae. Discovery of a malia, palaeogeography, premolar. The Early Cretaceous (Barremian, Wealden Group) which may or may not be of mammalian origin (pers. Wessex Formation of the Isle of Wight, southern England obs. -
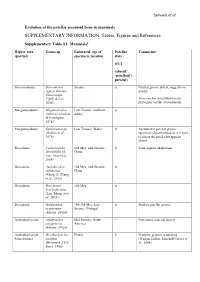
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

Two New Species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin Formations, Northeastern China
Historical Biology An International Journal of Paleobiology ISSN: 0891-2963 (Print) 1029-2381 (Online) Journal homepage: http://www.tandfonline.com/loi/ghbi20 Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China Nao Kusuhashi, Yuan-Qing Wang, Chuan-Kui Li & Xun Jin To cite this article: Nao Kusuhashi, Yuan-Qing Wang, Chuan-Kui Li & Xun Jin (2016) Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China, Historical Biology, 28:1-2, 14-26 To link to this article: http://dx.doi.org/10.1080/08912963.2014.977881 Published online: 01 Oct 2015. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ghbi20 Download by: [University of Sussex Library] Date: 01 October 2015, At: 18:24 Historical Biology, 2016 Vol. 28, Nos. 1–2, 14–26, http://dx.doi.org/10.1080/08912963.2014.977881 Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China Nao Kusuhashia*, Yuan-Qing Wangb, Chuan-Kui Lib and Xun Jinb aDepartment of Earth’s Evolution and Environment, Graduate School of Science and Engineering, Ehime University, Ehime 790-8577, Japan; bKey Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, P.R. China (Received 29 July 2014; accepted 14 October 2014) Two new gobiconodontid mammals, Gobiconodon tomidai sp. -

Science Journals
RESEARCH | REPORT PALEONTOLOGY the hyoid apparatus in monotremes and placen- tals, and in a modified condition in marsupials (for details, see the supplementary materials) New Jurassic mammaliaform (16–18). Based on the distinctly mammal-like morphol- sheds light on early evolution ogy of the hyoid elements of Microdocodon,we can now identify hyoid elements of several other mammaliaforms (16)(figs.S4toS9).TheJurassic of mammal-like hyoid bones haramiyidan Vilevolodon has preserved basihyal 1,2 3 4 and ceratohyal bones (fig. S6 and movie S2) (19). Chang-Fu Zhou *, Bhart-Anjan S. Bhullar *, April I. Neander , The Cretaceous eutriconodontan Yanoconodon 5† 4† Thomas Martin , Zhe-Xi Luo also has preserved hyoid elements (fig. S6) (20). Computed tomography (CT) scanning has re- We report a new Jurassic docodontan mammaliaform found in China that is preserved vealed the basihyal, thyrohyals, ceratohyals, and with the hyoid bones. Its basihyal, ceratohyal, epihyal, and thyrohyal bones have mobile epihyals of the trechnotherian Maotherium and joints and are arranged in a saddle-shaped configuration, as in the mobile linkage the multituberculate Sinobaatar of the Cretaceous of the hyoid apparatus of extant mammals. These are fundamentally different from (figs. S7 and S8 and movie S2) (16). Addition- the simple hyoid rods of nonmammaliaform cynodonts, which were likely associated ally, Sinobaatar has preserved stylohyal bones with a wide, nonmuscularized throat, as seen in extant reptiles. The hyoid apparatus (fig. S7), which is similar to the Cretaceous multi- provides a framework for the larynx and for the constricted, muscularized esophagus, tuberculate Kryptobaatar (21). We interpret this crucial for transport and powered swallowing of the masticated food and liquid in extant to mean that multituberculates have an integro- Downloaded from mammals. -

The Mesozoic Mesozoic Things to Think About
The Mesozoic Mesozoic Things to think about • Breakup of Pangea and its relationship to sealevel and climate • Dominance of reptiles • Origin of birds • Origin of mammals • Origin of flowers (angiosperms) • Expansion of insects • Life in the seas assumes an (almost) modern form 1 2 3 4 5 Triassic Period 248 to 206 Million Years Ago 6 The Connecticut River Valley 7 Dinosaur Footprints of the Connecticut Valley Edward Hitchcock Fossil Fish of the Connecticut Valley 8 Jurassic Period 206 to 144 Million Years Ago 9 Cretaceous Period 144 to 65 Million Years Ago 10 11 Mesozoic Ammonites 12 Cretaceous Heteromorph Ammonites Nipponites mirabilis Kamchatka, Russia Macroscaphites sp. Baculites sp. Didymoceras stevensoni Rudistid Bivalves: Jurassic- Cretaceous 13 Durania cornupastoris at Abu Roash, Western Desert near Gizah, Egypt Fringing Upper Cretaceous rudist reef reservoirs flanking basement highs, Augila oil field, eastern Libya Reef-forming rudist (Radiolites) from Sarvak Formation, Cenomanian, south Iran. 14 Biostrome of hippuritid rudists at Montagne des Cornes; Santonian, Pyrenees, France Rudistid Buildups 15 Biostrome of Vaccinites vesiculosus (Woodward, 1855); Campanian of Saiwan, Oman Monopleura marcida Albian, Viotía, Greece 16 Chalk 17 Pterosaurs Mosasaurs 18 Plesiosaurs Ichthyosaurs 19 A Dinosaur Family Tree (aka Phylogeny) 20 Sauropods Theropods 21 Dilong paradoxus Early Cretaceous, China A feathered tyrannosaurid? 22 A Dinosaur Family Tree (aka Phylogeny) Ornithopods (aka Hadrosaurs) 23 Thyreophorans (aka Ankylosaurs, etc) Margincephalians (aka Ceratopsians) 24 A Dinosaur Family Tree (aka Phylogeny) The Liaoning Fauna: An early Cretaceous Lagerstatten Some highlights: -- feathered dinosaurs -- preserved internal organs -- oldest placental mammal 25 The Liaoning Fauna The Liaoning Fauna Caudipteryx. Microraptor gui MICRORAPTOR zhaoianus. -

Dino Times! How Dinosaurs Lived What Did a Dinosaur’S World Look Like? Step Back in Time—About Eco-Quest 130 Million Years—To a Part of Eastern Asia Now Known As 1
Dino Times! How Dinosaurs Lived What did a dinosaur’s world look like? Step back in time—about Eco-Quest 130 million years—to a part of eastern Asia now known as 1. What similarities can you find Liaoning (“lee-ow-NING”), China. Here, in fossilized lakebeds, between the bird (Confuciusornis scientists have uncovered thousands of fossil remains, including sanctus) and Sinosauropteryx plants, insects, frogs, fish, small mammals, and even feathered prima or Dilong paradoxus dinosaurs! Together this collection creates a more complete pic- dinosaurs? Are you surprised by ture of what a dinosaur ecosystem (its community of plants, the similarities? Why or why not? animals, landscape, and weather) probably looked like. 2. Scientists believe that Dilong How did these animals and plants live together? Check out the paradoxus had a thin coat of feathers, but like its featherless illustrations below of Liaoning species discovered. Then answer cousin, the T. rex, it couldn’t fly. the questions in “Eco-Quest” to the right. What function do you think its feathers served? Think about what modern animals use feath- ers for. 3. Repenomamus giganticus was a carnivorous mammal about the B. Sinosauropteryx prima (“SIGN- size of a cat. From the plants and no-sore-AHP-ter-ix PREE-ma”), A. Eomaia scansoria (“ee- animals pictured here, what do feathered dinosaur oh-MY-ah SCAN-sor-ee- you suppose it ate? (Hint: It was- ah”), ancient mammal D. Dilong paradoxus n’t insects.) (“dee-LONG pair- 4. Scientists studied plant fossils uh-DOX-us”), feathered cousin and determined that the Liaoning of T. -

New Gobiconodontid (Eutriconodonta, Mammalia) from the Lower
第58卷 第1期 古 脊 椎 动 物 学 报 pp. 45–66 2020年1月 VERTEBRATA PALASIATICA figs. 1–5 DOI: 10.19615/j.cnki.1000-3118.190724 New gobiconodontid (Eutriconodonta, Mammalia) from the Lower Cretaceous Shahai and Fuxin formations, Liaoning, China KUSUHASHI Nao1 WANG Yuan-Qing2,3,4* LI Chuan-Kui2 JIN Xun2 (1 Department of Earth’s Evolution and Environment, Graduate School of Science and Engineering, Ehime University Matsuyama, Ehime 790-8577, Japan [email protected]) (2 Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences Beijing 100044, China * Corresponding author: [email protected]) (3 CAS Center for Excellence in Life and Paleoenvironment Beijing 100044, China) (4 College of Earth and Planetary Sciences, University of Chinese Academy of Sciences Beijing 100049, China) Abstract Eutriconodontans are one of the key members of mammals to our understanding of the evolution and transition of mammalian fauna in Asia during the Cretaceous. Two gobiconodontid and two triconodontid species have previously been reported from the upper Lower Cretaceous Shahai and Fuxin formations. Here we describe two additional eutriconodontans from the formations, Fuxinoconodon changi gen. et sp. nov. and ?Gobiconodontidae gen. et sp. indet. This new species is attributed to the Gobiconodontidae, characterized by having an enlarged first lower incisor, reduction in the number of incisors and premolariforms, proportionally large cusps b and c being well distant from cusp a on the molariforms, presence of a labial cingulid, and a unique mixed combination of molariform characters seen on either the first or the second, but not both, generations of molariforms in Gobiconodon.