Glaxosmithkline Annual Report 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNITED STATES SECURITIES and EXCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 SCHEDULE 13D Under the Securities Exchange Act of 1934 (Amendment No. 2)* GENOCEA BIOSCIENCES, INC. (Name of Issuer) Common Stock, Par Value $0.001 (Title of Class of Securities) 372427 10 4 (CUSIP Number) Victoria A. Whyte GlaxoSmithKline plc 980 Great West Road Brentford, Middlesex TW8 9GS England Telephone: +44 (0)208 047 5000 Name, Address and Telephone Number of Person Authorized to Receive Notices and Communications) July 30, 2015 (Date of Event which Requires Filing of this Statement) If the filing person has previously filed a statement on Schedule 13G to report the acquisition that is the subject of this Schedule 13D, and is filing this schedule because of §§240.13d-1(e), 240.13d-1(f) or 240.13d-1(g), check the following box. ☐ Note: Schedules filed in paper format shall include a signed original and five copies of the schedule, including all exhibits. See Rule.13d-7 for other parties to whom copies are to be sent. * The remainder of this cover page shall be filled out for a reporting person’s initial filing on this form with respect to the subject class of securities, and for any subsequent amendment containing information which would alter disclosures provided in a prior cover page. The information required on the remainder of this cover page shall not be deemed to be “filed” for the purpose of Section 18 of the Securities Exchange Act of 1934 (“Act”) or otherwise subject to the liabilities of that section of the Act but shall be subject to all other provisions of the Act (however, see the Notes). -

Governance and Remuneration 2014
Governance & remuneration reportStrategic In this section Our Board 72 Our Corporate Executive Team 76 Chairman’s letter 78 Corporate governance framework 79 Board report to shareholders Oversight and stewardship in 2014 and future actions 80 remuneration & Governance Leadership and effectiveness 82 Committee reports Audit & Risk 86 Nominations 92 Corporate Responsibility 94 Remuneration report Chairman’s annual statement 96 Annual report on remuneration 97 2014 Remuneration policy report 119 Financial statements Financial Investor information Investor GSK Annual Report 2014 71 Our Board Strategic reportStrategic Diversity Experience International experience Composition Tenure (Non-Executives) % % % % Scientific 19 Global 75 Executive 19 Up to 3 years 39 % % % % Finance 31 USA 100 Non-Executive 81 3-6 years 15 % % % % Industry 50 Europe 94 Male 69 7-9 years 23 % % % EMAP 63 Female 31 Over 9 years 23 Sir Christopher Gent 66 Skills and experience Chairman Sir Christopher has many years of experience of leading global businesses and a track record of delivering outstanding performance Governance & remuneration & Governance Nationality in highly competitive industries. He was appointed Managing Director British of Vodafone plc in 1985 and then became its Chief Executive Officer Appointment date in 1997 until his retirement in 2003. Sir Christopher was also a 1 June 2004 and as Chairman Non-Executive Director of Ferrari SpA and a member of the British on 1 January 2005 Airways International Business Advisory Board. Committee membership External appointments Corporate Responsibility Sir Christopher is a Senior Adviser at Bain & Co. Committee Chairman, Nominations, Remuneration and Finance Sir Philip Hampton 61 Skills and experience Chairman Designate Prior to joining GSK, Sir Philip chaired major FTSE 100 companies including J Sainsbury plc. -

The Everyday Lives of Recovering Heroin Users
The everyday lives of recovering heroin users Joanne Neale Sarah Nettleton Lucy Pickering The everyday lives of recovering heroin users The everyday lives of recovering heroin users Joanne Neale Sarah Nettleton Lucy Pickering Joanne Neale Professor of Public Health Faculty of Health & Life Sciences Oxford Brookes University Jack Straw’s Lane Marston Oxford OX3 0FL Email: [email protected] Sarah Nettleton Reader in Sociology Department of Sociology Wentworth College University of York Heslington York YO10 5DD Email: [email protected] Lucy Pickering Lecturer in Anthropology School of Social and Political Sciences University of Glasgow Adam Smith Building Glasgow G12 8RT Email: [email protected] Published by the RSA 8 John Adam Street London WC2N 6EZ +44 (0)20 7930 5115 Registered as a charity in England and Wales no. 212424 and in Scotland no. SCO 37784 Copyright © Joanne Neale 2012 The RSA is an enlightenment organisation devoted to finding innovative and practical solutions to today’s pressing social problems Cover photo: ‘Wings of butterfly,’ John Foxx. © Thinkstock Designed by Soapbox, www.soapbox.co.uk Printed and bound by CPI Antony Rowe www.theRSA.org Contents Acknowledgements 9 Foreword 10 Chapter 1: Setting the scene 14 Why read this book? 14 Why focus on recovery? 15 What is recovery? 15 A study of the everyday lives of recovering heroin users 17 Structure of the book 18 Chapter 2: Considering recovery 20 Introduction 20 Mapping services and support 20 Factors that can encourage and sustain recovery efforts -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -

Annual Review 2005
GS2184_Review_A\W2.qxd 7/3/06 4:58 pm Page fc1 Annual Review 2005 human being Do more, feel better, live longer GS2184_Review_A\W2.qxd 9/3/06 1:28 pm Page ifc2 01 An interview with Sir Christopher Gent, Chairman, and JP Garnier, Chief Executive Officer 05 Tachi Yamada, Chairman, Research & Development, Pharmaceuticals 06 Jean Stéphenne, President and General Manager, GSK Biologicals 08 John Clarke, President, Consumer Healthcare 11 David Stout, President, Pharmaceutical Operations 14 Performance highlights 15 Business operating review 18 The Board 19 The Corporate Executive Team 20 Summary remuneration report 23 Corporate governance 24 Responsibility statements 25 Summary financial statements 26 Summary information under US GAAP 27 Shareholder information 29 Chairman and CEO’s closing letter JP Garnier (left) and Sir Christopher Gent (right) GS2184_Review_A\W2.qxd 7/3/06 5:01 pm Page 01 “Discovering important medicines, eradicating diseases, improving the quality of people’s lives and making medicines available to a greater number of people. This is what we do – and what we do matters to people.” JP Garnier, Chief Executive Officer An interview with Sir Christopher Gent, Chairman and JP Garnier, Chief Executive Officer 2005: a year of success and progress “Thanks to the efforts of our employees around the company’s pipeline is one of the largest and most world, 2005 was a very successful year for GSK,” says promising in the industry, with 149 projects in clinical JP Garnier, Chief Executive Officer. “Not only was it development (as at the end of February 2006), our best year ever from a financial standpoint, we also including 95 new chemical entities (NCEs), 29 product made substantial progress with our pipeline of line extensions (PLEs) and 25 vaccines. -

Other Statutory Disclosures Continued
Other statutory disclosures continued Strategic reportGroup companies Governance & remuneration Financial statements In accordance with Section 409 of the Companies Act 2006 a full list of subsidiaries, associates, joint ventures and joint arrangements, the country of incorporation and effective percentage of equity owned, as at 31 December 2015 are disclosed below. Unless otherwise stated the share capital disclosed comprises ordinary shares which are indirectly held by GlaxoSmithKline plc. All subsidiary companies are resident for tax purposes in their country of incorporation unless otherwise stated. Country of Effective % % Held by Name incorporation Ownership Security Class of Share Wholly owned subsidiaries 1506369 Alberta ULC Canada 100 Common 100 Action Potential Venture Capital Limited England & Wales 100 Ordinary 100 Adechsa GmbH Switzerland 100 Ordinary 100 Affymax Research Institute United States 100 Common 100 Alenfarma – Especialidades Farmaceuticas, Limitada (iv) Portugal 100 Ordinary Quota 100 Allen & Hanburys Limited (iv) England & Wales 100 Ordinary 100 Allen & Hanburys Pharmaceutical Nigeria Limited Nigeria 100 Ordinary 100 Allen Farmaceutica, S.A. Spain 100 Ordinary 100 Allen Pharmazeutika Gesellschaft m.b.H. Austria 100 Ordinary 100 Aners S.A (iv) Argentina 100 Non-endorsable Nominative Ordinary 100 Barrier Therapeutics, Inc. United States 100 Common 100 Beecham Group p l c England & Wales 100 20p Shares 'A'; 5p Shares B 100 Beecham Pharmaceuticals (Pte) Limited Singapore 100 Ordinary 100 Beecham Pharmaceuticals S.A (iv) (vi) Ecuador 100 Nominative 100 Beecham Portuguesa-Produtos Farmaceuticos e Quimicos, Lda Portugal 100 Ordinary Quota 100 Beecham S.A. (iv) Belgium 100 Ordinary 100 Biddle Sawyer Limited India 100 Equity 100 Biovesta Ilaçlari Ltd. Sti. Turkey 100 Nominative 100 Burroughs Wellcome & Co (Australia) Pty Limited (iv) (vi) Australia 100 Ordinary 100 Burroughs Wellcome & Co (Bangladesh) Limited Bangladesh 100 Ordinary 100 Burroughs Wellcome International Limited England & Wales 100 Ordinary 100 Caribbean Chemical Company, Ltd. -
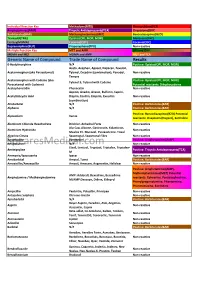
Cross Reaction Guide
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

2005 GSK Corporate Responsibility Report
2005 GSK Corporate Responsibility Report Introduction 1 Introduction Page Our contribution to society 1 About this report 1 CEO and Chairman’s letter 3 Managing corporate responsibility 4 Why CR is important to GSK 4 CR governance 4 Our CR principles 6 Stakeholder engagement 7 Stakeholder feedback 7 How we are responding 9 Government and external affairs 10 Membership of trade associations 10 US lobbying expenditures 10 Political donations 10 Our position on key issues 11 Patient advocacy 11 2 Access to medicine 3 Research 4 Ethical conduct 5 Employment practices 6 Human rights 7 Environment 8 Community investment 9 Data summary 1 1 GSK CORPORATE RESPONSIBILITY REPORT 2005 Introduction Welcome to GSK’s Corporate Responsibility Report 2005. This report explains our approach to the wide range of social, ethical and environmental issues associated with our business and reports our performance in 2005. The full web-based report is available on www.gsk.com GSK is a research-based pharmaceutical company, with operations in 119 countries. We make prescription medicines, vaccines, over-the-counter medicines, and consumer healthcare products. Our business accounts for 6.3% of the world’s pharmaceutical market. We have strong positions in several therapeutic areas including anti-infectives, asthma, cancer, cardiovascular, depression, diabetes, HIV/AIDS and urology. For an overview of our business, see our Annual Report. OUR CONTRIBUTION TO SOCIETY This report explains our approach to the significant In the last 80 years, medicines and vaccines have corporate responsibility issues for our business, including: transformed millions of lives. They have helped to increase • Access to medicines – how we make our medicines life expectancy and lowered death rates from conditions accessible to poor patients in developed and developing such as heart disease, stroke and cancer. -

In This Section
Strategic report In this section Chairman’s statement 2 CEO’s review 4 Business overview 6 The global context 8 Our business model 12 Our strategic priorities 14 How we performed 16 Risk management 18 Grow 20 Deliver 32 Simplify 44 Our financial architecture 48 Responsible business 50 Financial review 58 Strategic report Chairman’s statement Chairman’s statement To shareholders The value of the significant changes that have been made in recent years is evidenced in our performance this year “ Since Sir Andrew became It is clear from the following pages that Through the Audit & Risk Committee, we the Group made good progress against oversee the issues and challenges faced by CEO, the company has its strategy in 2013. management, and encourage the creation of an environment in which GSK can achieve The Board believes the business is seeing returned £30 billion its strategic ambitions in a responsible and the benefits of the significant changes the sustainable manner. to shareholders.” management team has driven over recent years to deliver sustainable growth, reduce risk and I have no doubt that commercial success is enhance returns to shareholders. directly linked to operating in a responsible way and which meets the changing expectations of The notably strong performance from the society. In this respect, the company continues R&D organisation in 2013 – with six major to adopt industry-leading positions on a range new product approvals in areas including of issues. respiratory disease, HIV and cancer – is critical to the longer-term prospects of the The announcement of plans during 2013 to Group. -

With Nicotine Replacement Therapies in Smoking Cessation Vol
Health Technology Assessment Health Technology Health Technology Assessment 2008; Vol. 12: No. 2 2008; 12: No. 2 Vol. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis D Wang, M Connock, P Barton, A Fry-Smith, P Aveyard and D Moore Feedback The HTA Programme and the authors would like to know your views about this report. The Correspondence Page on the HTA website (http://www.hta.ac.uk) is a convenient way to publish your comments. If you prefer, you can send your comments to the address below, telling us whether you would like us to transfer them to the website. We look forward to hearing from you. February 2008 The National Coordinating Centre for Health Technology Assessment, Mailpoint 728, Boldrewood, Health Technology Assessment University of Southampton, NHS R&D HTA Programme Southampton, SO16 7PX, UK. HTA Fax: +44 (0) 23 8059 5639 Email: [email protected] www.hta.ac.uk http://www.hta.ac.uk ISSN 1366-5278 HTA How to obtain copies of this and other HTA Programme reports. An electronic version of this publication, in Adobe Acrobat format, is available for downloading free of charge for personal use from the HTA website (http://www.hta.ac.uk). A fully searchable CD-ROM is also available (see below). Printed copies of HTA monographs cost £20 each (post and packing free in the UK) to both public and private sector purchasers from our Despatch Agents. Non-UK purchasers will have to pay a small fee for post and packing. -

PDF (Irish Pharmacist January 2020)
EXCLUSIVE COVERAGE FROM THE RECENT IRISH MEDICATION SAFETY NETWORK ANNUAL CONFERENCE ABOUT-TURN WE LOOK AT WHAT MIGHT BE NEXT FOR PHARMACISTS FOLLOWING THE REVERSAL IN PROPOSED FEE CUTS DUMB AND DUMBER? ARE PHARMACISTS A LITTLE LACKING IN GREY MATTER WHEN IT COMES TO PRICING, ASKS FINTAN MOORE PLUS CLINICAL CONTENT: ASTHMA ❋ COPD ❋ EYE CARE ❋ SMOKING CESSATION TENDER CARE AT Every Change For topical use only. Cleanse and dry the affected area before applying. A copy of the summary of product characteristics is available upon request. The active ingredient in Caldesene Medicated Powder is Calcium Undecylenate 10% w/w, 20g, 55g, 100g pack size. For supply through general sale. PA 126/152/1 PA Holder: Clonmel Healthcare Ltd., Waterford Road, Clonmel, Co. Tipperary. Date Prepared: October 2019. 2019/ADV/CAL/150H NEW TREATS HEARTBURN AND ACID REFLUX. ONE TABLET PER DAY. LASTS 24 HOURS. AVAILABLE IN PACKS OF 7s AND 14s. Marketed by CCF:22656 Date of preparation: (10-19) ABBREVIATED PRESCRIBING INFORMATION Product Name: Emazole Control 20 mg Gastro-Resistant Tablets Composition: Each tablet contains 20 mg esomeprazole (as magnesium dihydrate). Description: Light pink oval film coated tablet. Indication(s): Proton Pump Inhibitor (PPI): Short-term treatment of reflux symptoms (e.g. heartburn and acid regurgitation) in adults. Dosage: Swallow tablets whole with liquid, do not chew or crush. Disperse in half a glass of non-carbonated water if difficulty in swallowing. Stir until tablets disintegrate, drink liquid with pellets immediately or within 15 min, or administer through a gastric tube. Do not chew or crush pellets. Adults: The recommended dose is 20 mg esomeprazole (one tablet) per day. -
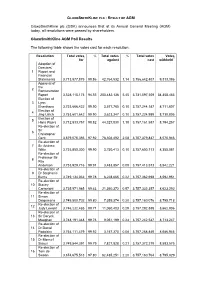
Voting/Poll Results
GLAXOSMITHKLINE PLC - RESULT OF AGM GlaxoSmithKline plc (GSK) announces that at its Annual General Meeting (AGM) today, all resolutions were passed by shareholders. GlaxoSmithKline AGM Poll Results The following table shows the votes cast for each resolution: Resolution Total votes % Total votes % Total votes Votes for* against cast withheld** Adoption of Directors’ 1 Report and Financial Statements 3,713,877,875 98.86 42,764,532 1.14 3,756,642,407 9,313,386 Approval of the 2 Remuneration Report 3,528,115,173 94.55 203,482,136 5.45 3,731,597,309 34,358,483 Election of 3 Lynn Elsenhans 3,753,666,422 99.90 3,577,765 0.10 3,757,244,187 8,711,607 Election of 4 Jing Ulrich 3,753,601,642 99.90 3,623,347 0.10 3,757,224,989 8,730,805 Election of 5 Hans Wijers 3,712,833,757 98.82 44,327,830 1.18 3,757,161,587 8,794,257 Re-election of Sir 6 Christopher Gent 3,679,075,355 97.92 78,304,492 2.08 3,757,379,847 8,575,946 Re-election of 7 Sir Andrew Witty 3,753,850,300 99.90 3,750,413 0.10 3,757,600,713 8,355,081 Re-election of Professor Sir 8 Roy Anderson 3,753,929,716 99.91 3,483,857 0.09 3,757,413,573 8,542,221 Re-election of 9 Dr Stephanie Burns 3,749,134,033 99.78 8,228,665 0.22 3,757,362,698 8,592,951 Re-election of 10 Stacey Cartwright 3,735,971,985 99.43 21,360,372 0.57 3,757,332,357 8,623,292 Re-election of 11 Simon Dingemans 3,749,800,702 99.80 7,359,374 0.20 3,757,160,076 8,795,718 Re-election of 12 Judy Lewent 3,746,232,485 99.71 11,060,403 0.29 3,757,292,888 8,662,906 Re-election of 13 Sir Deryck Maughan 3,748,191,348 99.76 9,051,199 0.24