First Record of the North American Cryptic Invader Ferrissia Fragilis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bourj Hammoud, Lebanon
NABAA NEIGHBOURHOOD PROFILE & STRATEGY Bourj Hammoud, Lebanon March 2017 1 FOREWORD “Building development to address the emergency” is Bourj Hammoud is a municipal district with one of the most the philosophy behind the intervention that the Italian diverse populations in the country. It is also one of the most Agency for Development Cooperation (AICS) is financing active and vibrant industrial and economic hubs. and realising together with the United Nations Human Settlements Programme (UN-Habitat). Being on the northern boundary of the capital, this swampy area was home to a few farming families, until survivors of “Building development” because working to strengthen the Armenian Genocide were settled there by the authorities “Building development to address the emergency” isinfrastructure the philosophy and behind enhance the public services in the marginal of the day. These and their descendants inhabited the area intervention that the Italian Agency for Development Cooperationareas of Lebanese (AICS) is financingcities is, in the medium and long term, a and constructed residences and businesses there. The area and realizing together with the United Nation Human principalSettlements road Programme to a better (UN quality of life for vulnerable groups became a prosperous industrial and commercial zone that Habitat). of the population. soon turned into an attraction point for job-seeking people from around the country and abroad. Building development because working to strengthen “Addressinfrastructures the andemergency” enhance since the rapid and constant public services in the marginal areas of Lebanese cities urbanisationis, in the mid and of long the period, country’s cities is a factor associated Today, this densely populated area is facing several challenges the principal road to guarantee a better quality of lifewith for the social vulnerable vulnerability groups ofin many metropolitan areas. -

Beirut Riverless
This resulted in “killing” the river by disconnecting it •Provisioning Services, where the river provides fresh from its larger natural ecosystem, and “killing” it again water for irrigation in the area of Daychounieh and BEIRUT by disconnecting it from the human communities that potable water to the city through the Roman aqueducts, used to enjoy it and care for it. as well as transporting sediments, organisms and nutrients. RIVERLESS The river thus became a no-man’s-land and a dumping Adib DADA ground affected by every crisis the city has gone through: •Regulating Services, where the basin treats and stores Founder and Lead Architect at theOtherDada [email protected] the dumping of slaughterhouse waste when the illegal water, controls erosion to mitigate the impact of floods slaughterhouses scandal came to light; the dumping and storms, and filters waste through natural processes. Beirut RiverLESS aims to address the deterioration of the of garbage during the nine-month long garbage crisis; •Supporting Services, where the river offers food, Beirut River and its negative impact on the surrounding a regular dumping ground for textile dyes, which turn shelter, and water to living organisms, and also acts communities and environment, by developing a holistic the river bright red; even an unloved crocodile pet was as a vital migratory path for more than 70,000 soaring response plan for the Beirut River Watershed. The goal is dumped and found living there. Not to mention the raw birds, not to mention that its valley is officially listed as to bring Beirut River back to life by following a Landscape sewage infrastructure that is directly connected to the an Important Bird Area [IBA]2. -

Urban Forests Provide Hope for the Beirut River Writer: Adib Dada (
Stories (https://amwaj-alliance.com/category/stories/) Urban forests provide hope for the Beirut River Writer: Adib Dada (https://amwaj-alliance.com/author/adib-dada/) The Beirut River and the surrounding neighborhoods have long suered from short-sighted policy and blatant mismanagement. The ecosystem was disrupted, and natural habitats have been destroyed. The #BeirutRiverForest project consists of planting trees on the banks of the river, as well as engaging with local associations, schools, and companies in developing a blueprint for the potential ‘green corridor’ of Beirut to emerge. Aerial view of the Beirut River above Sin el-Fil area (Source: theOtherDada) This area used to be the floodplain of the Beirut River, a fresh water source for the Roman city of Berytus (currently Beirut) and earlier civilizations. The ecosystem was a riparian one, of which we can still see a few kilometers. The area was slowly turned into farmland, and planted with aggressively invasive species, such as eucalyptus, that dried up the land. In the early 20th century, several waves of immigration resulted in informal and formal settlements in those farmlands. For most of the 20th century, the banks of the river had been natural, providing the communities with ecosystem services such as fresh food and water, helping to regulate temperatures and snowmelt, supporting humans and wildlife habitats, and providing a space for recreation activities for people from either side of the river to enjoy. The Armenian water festival Vardavar and the Muslim Eid were celebrated together with dierent communities. Picnics, fishing, and boating served to bring people together as well. -

Planning a Sectarian Topography Revisiting Michel Ecochard’S Master Plans for Beirut Between 1941-1964
Planning a Sectarian Topography Revisiting Michel Ecochard’s Master Plans for Beirut between 1941-1964 by Ali Khodr Bachelor of Architecture (BArch.) American University of Beirut, 2015 SUBMITTED TO THE DEPARTMENT OF ARCHITECTURE IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTERS OF SCIENCE IN ARCHITECTURE STUDIES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 2017 © Ali Khodr. All Rights Reserved The author hereby grants to MIT the permission to repro- duce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature of Author: __________________________________________________________________________ Department of Architecture May 25, 2017 Certified by: __________________________________________________________________________ Professor Nasser Rabbat Professor of the History of Architecture & Aga Khan Professor Thesis Supervisor Accepted by: __________________________________________________________________________ Professor Sheila Kennedy Professor of Architecture Chair of the Department Committee on Graduate Students Planning a Sectarian Topography Revisiting Michel Ecochard’s Master Plans for Beirut between 1941-1964 Ali Khodr Thesis Committee Nasser Rabbat, MArch, PhD Aga Khan Professor, Professor of the History of Architecture Director, Aga Khan Program Supervisor James Wescoat, PhD Aga Khan Professor, Professor of Urban Studies and Planning Associate Head, Department of Architecture Reader Hashim Sarkis, PhD Dean, -

Beirut Riverless
RIVER, NAHR, RÍO Adib Dada The Beirut River, the city’s largest open river, emerges dams, canals, aqueducts, and tunnels, used for pota- thirty kilometers inland from two points in Hammana ble water. Parts of the aqueduct ruins still remain in and Tarchiche at an altitude of around 1900 meters. the Daychounie area, in the valley between Mansou- Throughout time, the river has been used as a water re- rieh and Hazmieh. source for drinking and irrigation. It also once had an important role as a space for recreational activities. In During the nineteenth century and first half of the 1968, the river was transformed from a natural, healthy twentieth century, the river water was mainly used for and performing ecosystem to a canalized infrastruc- agricultural purposes. In 1934, during the French man- ture, becoming an open sewer of domestic and indus- date period, the French built a dam in the Daychounie trial wastewater, highly polluted and posing numerous area in an attempt to modernize agriculture. However, health risks to its neighbors. The river also lost its rec- sediments filled the storage capacity of the dam, not reational and social function as people no longer had only ruining the efficiency of the structure but also af- access to it. fecting the water flow of the river and its ecosystem. Beirut RiverLESS, a project initiated by theOtherDada, During the 1940s, the Armenian refugees who originally aims to address the deterioration of the Beirut River located in the Quarantina area were relocated to Achra- and its negative impact on the surrounding communi- fieh and Bourj Hammoud and settled on both sides of ties and environment by developing a holistic response the river. -
Assessment of Water Resources and Water Sector in Lebanon
NO.11 SUPPLEMENT 3 ⏐ SEPTEMBER 2004 ⏐ ENGLISH VERSION Lebanon Water as a human right: Assessment of water resources and water sector in Lebanon By Ali Darwish, Green Line Association, Lebanon Global Issue Papers, No. 11 Water as a human right: The understanding of water in the Arab countries of the Middle East – A four country analysis Published by the Heinrich Böll Foundation © Heinrich Böll Foundation 2004 All rights reserved The following paper does not necessarily represent the views of the Heinrich Böll Foundation. - 108 - Summary Lebanon is the richest country in the Middle Eastern region in its water resources. The country receives about 9 billion cubic metres of rainfall more than half of which is lost as runoff and evapotranspiration as a result of the topography and the length of the dry season. Despite this, Lebanon suffers from a shortage of water for irrigation, domestic and industrial uses. This deficit is mainly due to a historical negligence on the part of the government before the 1975 conflict and the lack of planning in the rehabilitation after 1991. Although Lebanon has accessed the Convent on Economic Social and Cultural Rights as early as 1976, and has signed and ratified most other related conventions, the ten- year master plan for the water resources management was only launched in the year 2000. Until date, the works were initiated on only one dam of the more than 30 planned dams and hill lakes. The lack of financial resources in a heavily indebted country and conflicting interests of politicians are the major barriers facing the implementation of the plan. -

Lebanon's Birds
State of Lebanon’s Birds and IBAs State of Lebanon’s Birds and IBAs State of Lebanon’s Birds and IBAs Project’s name : Mainstreaming Conservation of Migratory Soaring Birds into Key Productive Sectors along the Rift Valley/Red Sea Flyway Executing Organization : Ministry of Environment Managing Organization : United Nations Development Programme Funding Organization : Global Environment Facility Regional Technical Partner : BirdLife International Sub-Contracting Technical Partner : Society for the Protection of Nature in Lebanon Collated & Edited by : Assad Adel Serhal & Bassima Chafic Khatib Printed by : Dar Bilal for Printing and Publishing The information, results, interpretations and conclusions contained in this book Beirut, Lebanon are those of the authors, and do not necessarily represent the viewpoint of the Ministry of Environment or the United Nations Development Program Tel/fax 00961 1 852 869 00961 3 900879 Layout & Design : Ali Kamal Eddine Cover Illustrations : Husein Ali Zorkot All rights are reserved for the authors State of Lebanon’s Birds and IBAs State of Lebanon’s Birds and IBAs Table of Content Foreword 8 2.7.1.1 Sedentary Species 126 Acknowledgment 10 2.7.1.2 Summar Breeding Species 139 Executive Summary 12 2.7.1.3 Vagrant Species 149 List of Abbreviations 14 2.7.1.4 Passage Migrants, Winter Visitors, and Summar Visitors 153 2.7.1.5 Threatened Species 179 Section One: Important Bird Area Programme (Sites) 2.8 Detailed Status of Migratory Soaring & Semi-Soaring Birds in Lebanon 180 1.1 Definition of Important Bird -
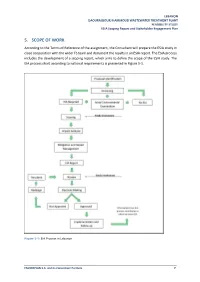
5. Scope of Work
LEBANON DAOURA/BOURJ HAMMOUD WASTEWATER TREATMENT PLANT FEASIBILITY STUDY ESIA Scoping Report and Stakeholder Engagement Plan 5. SCOPE OF WORK According to the Terms of Reference of the assignment, the Consultant will prepare the ESIA study in close cooperation with the wider FS team and document the results in an ESIA report. The ESIA process includes the development of a scoping report, which aims to define the scope of the ESIA study. The EIA process chart according to national requirements is presented in Figure 5-1. Figure 5-1: EIA Process in Lebanon ENVIROPLAN S.A. and its Consortium Partners 7 LEBANON DAOURA/BOURJ HAMMOUD WASTEWATER TREATMENT PLANT FEASIBILITY STUDY ESIA Scoping Report and Stakeholder Engagement Plan The following provides an overview of the overall sections and subsections of an ESIA process and is structured in accordance with MOE Decision 261/1 for 2015. 5.1. Policy, Legal and Administrative Framework 5.1.1. Authorities and Institutions Several public institutions will be involved in the different phases of the proposed extension and upgrade of the pre-treatment WWTP in Daoura/Bourj Hammoud. These authorities and institutions are listed below and their relevant role and mandate will be described in the ESIA: • Council for Development and Reconstruction • Ministry of Environment • Ministry of Energy and Water • Directorate General for Urban Planning (DGUP) / Higher Council for Urban Planning (HCUP) • Beirut and Mount Lebanon Water Establishment • Ministry of Public Health (MOPH) • Municipality of Bourj Hammoud -

Explosions Responsebeirut Port
BEIRUT PORT RESPONSE EXPLOSIONS Bourj Hammoud Municipality Rapid Building-level Damage Assessment Municipality of Bourj Hammoud and UN-Habitat October 2020 Working Version With support from Citation format: Municipality of Bourj Hammoud and UN-Habitat (2020), Beirut: UN-Habitat Lebanon. Copyright © 2020 Municipality of Bourj Hammoud and UN-Habitat. All rights reserved. Cover photo: © UN-Habitat (2020). PARTNERS Academic institutions: International and local organisations: CREDITS UN-Habitat Lebanon Authors: Lady Habchy; Wael Sinno. GIS and IM: Charbel Abou Chakra. Data Analysis/Visualization and Report Production/Design Layout: Georges Abi Sleiman; Ruba Abou El Houda. Editor: Suzanne Maguire; Taina Christiansen. Municipality of Bourj Hammoud Georges Krikorian. Garine Balian. Lila Kendirjian. TABLE OF CONTENTS Introduction 1 Purpose of report Administrative boundaries and assessment zones Background and context Methodology 3 Zoning Visual inspection steps for surveyors PURPOSE OF REPORT Habitability based on signs of damage Work progress milestones Findings 8 Coding of buildings for damage and habitability Assessment findings Next Steps 10 Annex 11 Photo gallery ADMINISTRATIVE BOUNDARIES AND ASSESSMENT ZONES AND ASSESSMENT BOUNDARIES ADMINISTRATIVE INTRODUCTION The Port of Beirut explosions of 4th August 2020, situated the extent of damage and particularly structural impacts, within the boundaries of the Municipality of Beirut (MoB) immediately identifying buildings at risk of collapse and and in close proximity to the neighbouring Municipality -

MGM Construction S.A.L Company Profile 2015 RAMCO
MGM Construction S.A.L Airport Avenue, Kayan Bldg, 2nd floor, Beirut, Lebanon Tel/Fax: 961 (5) 951 554, Mob: 961 (3) 329 862 E-mail: [email protected] Company Profile 2015 Presented to: RAMCO WWW.MGMSAL.COM INTRODUCTION Airport Avenue, Kayan Bldg, 2nd floor, Beirut, Lebanon, website: www.mgmsal.com Tel/Fax: 961 (5) 951 554, Mob: 961 (3) 329 862, E-mail: [email protected] Dear Sirs, We are pleased to present to you a brief profile of our company. MGM Construction is a Lebanese contracting company established and registered in 2010 under the number 2019170 in the registry of commerce at Baabda. MGM was founded by Eng. Georges Kenaan, a geotechnical engineer with over 30 years of experience in geotechnical engineering in the US and established in Lebanon since 1998. Since its foundation MGM has strived to provide its clients with high-quality service in the Geotechnical field. These services include Design, Technical assistance and Execution of Special works. MGM is specialized in the “Design & Build” of Special Foundation and Shoring works such as piling, diaphragm wall, micro-piling, temporary and permanent anchoring, nailing, shotcreting, cement grouting, dewatering, soil investigation, cavity probing and filling, etc… MGM has been involved in the execution of numerous projects of residential, commercial and industrial nature which are summarized in the following profile. Our company has the experience, the technical know-how, the tools and the personnel to undertake important works such as deep excavations in urban areas, deep foundations, slope reinforcement, soil improvement, soil investigation campaigns and other soil related works. -

The Case of Beirut, Lebanon by Mona Fawaz and Isabelle Peillen
The case of Beirut, Lebanon by Mona Fawaz and Isabelle Peillen Contact: Mona Fawaz Source: CIA factbook Massachusetts Institute of Technology AUB Department of Architecture Bliss Street Lebanon Tel. +961 1381 281 Fax +961 1820 472 [email protected] [email protected] PREFACE On the Difficulties of Mapping Slums in Beirut We attached a great deal of importance to mapping land speculation and made access more costly. the slums in Beirut, especially because we believed in However, shortly after 1975, most of the residents of the the need for producing this document at a time when north-eastern suburban slums of Beirut were evicted there is little if any public debate around the question of from their houses and many started squatting in various informal settlements in Beirut. Nevertheless, we faced areas of the southern suburbs, in expensive sea-front many difficulties in achieving our task. In this preface to beach resorts, empty green lots, or institutional build- the report, we describe some of these difficulties ings. Their numbers, compounded by an extensive rural because we believe that they are important for under- to urban migration fuelled by the two Israeli invasions standing the slums of Beirut. (1978 and 1982) and the Israeli occupation of South A particular challenge to undertaking the mapping Lebanon (1978-2000), led to the transformation of most slums in Beirut is the absence of a unified understand- of the open spaces of the southern suburbs of Beirut ing of the city. Indeed, during the years of the civil war into large slums. Today, it is the southern suburbs of (1975-1990), the city was divided into two sections, Beirut that carry the label of “illegal settlements” in most each run by one or several antagonistic groups. -

Beirut, Lebanon
Beirut, Lebanon Introduction Beirut: Its history dates back over 5,000 years. Under the city’s program. Massive privatized public investment was coupled with values which were the founding principles of such a diverse com- downtown area lay remnants of Ottoman, Mamluk, Crusader, Ab- macro-economic policies designed to stimulate private local and munity damaging the entire strata of urban socio-economic val- bassid, Omayyad, Byzantine, Roman, Persian, Phoenician and foreign investment. ues. The city has become a bourgeois ghetto catering to only 5% - of the Lebanese population .( LE SCHÉMA DIRECTEUR DE LA ony, only to be destroyed later by a triple catastrophe of earth- The development company, SOLIDER was established within the RÉGION MÉTROPOLITAINE DE BYEROUTH (SDRMB, 1986), framework of Law 117 of 1991 which regulates Lebanese real es- The SDRMB plan is considered the reference for the reconstruc- until the Crusaders took over in 1110. Following the Crusaders tate companies aiming at the reconstruction of war-damaged areas, tion of the Beirut metropolitan region today. were the Mamlukes and the Turks, and after World War I there was a French mandate period ended in 1943. There after Beirut wit- DIRECTEUR” catering to the private upper class which is 5% of nessed numerous local and regional wars of civil and uncivil hostil- the population and negating the rest of the Lebanese population. Based on a Master Plan which optimizes the site’s natural assets ities coupled with a twenty years of Israeli occupation ended year “More than half of Lebanon’s 3 million people live below the pover- and draws on its rich heritage, the project aims at creating a mod- 2000 and thirty years of Syrian control ended 2005.