Isakos/Esska Standard Terminology, Definitions, Classification and Scoring Systems for Arthroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supracondylar Femoral Extension Osteotomy and Patellar Tendon Advancement in the Management of Persistent Crouch Gait in Cerebral Palsy
Original Article Supracondylar femoral extension osteotomy and patellar tendon advancement in the management of persistent crouch gait in cerebral palsy Sakti Prasad Das, Sudhakar Pradhan, Shankar Ganesh1, Pabitra Kumar Sahu, Ram Narayan Mohanty, Sanjay Kumar Das ABSTRACT Background: Severe crouch gait in adolescent cerebral palsy is a difficult problem to manage. The patients develop loading of patellofemoral joint, leading to pain, gait deviation, excessive energy expenditure and progressive loss of function. Patella alta and avulsion of patella are the other complications. Different treatment options have been described in the literature to deal with this difficult problem. We evaluated outcome of supracondylar femoral extension osteotomy (SCFEO) and patellar tendon advancement (PTA) in the treatment of crouch gait in patients with cerebral palsy. Materials and Methods: Fourteen adolescents with crouch gait were operated by SCFEO and PTA. All subjects were evaluated pre and postoperatively. Clinical, radiographic, observational gait analysis and functional measures were included to assess the changes in knee function. Results: Cases were followed up to 3 years. The patients walked with increased knee extension and improvement in quadriceps muscle strength. Knee pain was decreased and improvements in functional mobility and radiologic improvement were found. Conclusion: SCFEO and PTA for adolescent crouch gait is effective in improving knee extensor strength, reducing knee pain and improving function. Key words: Crouch gait, patellar -

Ankylosing Spondylitis
Page 1 of 4 Ankylosing Spondylitis Ankylosing spondylitis (AS) is a form of arthritis. It mainly affects the lower back. Other joints and other parts of the body are sometimes affected. Treatment includes regular exercise and anti-inflammatory drugs. The severity of AS varies from mild to severe. It is mild or moderate in most cases. What is ankylosing spondylitis? Spondylitis means inflammation of the spine. Ankylosing is a word that describes bones that tend to join together (fuse) across a joint. In ankylosing spondylitis (AS), the discs and ligaments of the lower spine become inflamed. The discs and ligaments are the strong tissues that connect the spinal bones (vertebrae) together. The joints between the lower spine and the pelvis (the sacro-iliac joints), and the small facet joints between the vertebrae are also commonly affected. Inflammation around the lower spine that persists long-term can cause scarring. This may, over time, cause some of the vertebrae in the spine to fuse together. In some cases, inflammation occurs in other joints and in other parts of the body outside of the spine (detailed below). Who gets ankylosing spondylitis? AS usually develops in teenagers or young adults. It rarely first develops after the age of 40. It is three times more common in men than women. There may be a family history with two or more members of a family being affected. About 1 in 1000 people in the UK have AS. What causes ankylosing spondylitis? The cause of AS is not known. There is a strong genetic (hereditary) part. Something may 'trigger' AS to develop in people who have an inherited tendency to have it. -

Netter's Musculoskeletal Flash Cards, 1E
Netter’s Musculoskeletal Flash Cards Jennifer Hart, PA-C, ATC Mark D. Miller, MD University of Virginia This page intentionally left blank Preface In a world dominated by electronics and gadgetry, learning from fl ash cards remains a reassuringly “tried and true” method of building knowledge. They taught us subtraction and multiplication tables when we were young, and here we use them to navigate the basics of musculoskeletal medicine. Netter illustrations are supplemented with clinical, radiographic, and arthroscopic images to review the most common musculoskeletal diseases. These cards provide the user with a steadfast tool for the very best kind of learning—that which is self directed. “Learning is not attained by chance, it must be sought for with ardor and attended to with diligence.” —Abigail Adams (1744–1818) “It’s that moment of dawning comprehension I live for!” —Calvin (Calvin and Hobbes) Jennifer Hart, PA-C, ATC Mark D. Miller, MD Netter’s Musculoskeletal Flash Cards 1600 John F. Kennedy Blvd. Ste 1800 Philadelphia, PA 19103-2899 NETTER’S MUSCULOSKELETAL FLASH CARDS ISBN: 978-1-4160-4630-1 Copyright © 2008 by Saunders, an imprint of Elsevier Inc. All rights reserved. No part of this book may be produced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording or any information storage and retrieval system, without permission in writing from the publishers. Permissions for Netter Art figures may be sought directly from Elsevier’s Health Science Licensing Department in Philadelphia PA, USA: phone 1-800-523-1649, ext. 3276 or (215) 239-3276; or e-mail [email protected]. -

Acute < 6 Weeks Subacute ~ 6 Weeks Chronic >
Pain Articular Non-articular Localized Generalized . Regional Pain Disorders . Myalgias without Weakness Soft Tissue Rheumatism (ex., fibromyalgia, polymyalgia (ex., soft tissue rheumatism rheumatica) tendonitis, tenosynovitis, bursitis, fasciitis) . Myalgia with Weakness (ex., Inflammatory muscle disease) Clinical Features of Arthritis Monoarthritis Oligoarthritis Polyarthritis (one joint) (two to five joints) (> five joints) Acute < 6 weeks Subacute ~ 6 weeks Chronic > 6 weeks Inflammatory Noninflammatory Differential Diagnosis of Arthritis Differential Diagnosis of Arthritis Acute Monarthritis Acute Polyarthritis Inflammatory Inflammatory . Infection . Viral - gonococcal (GC) - hepatitis - nonGC - parvovirus . Crystal deposition - HIV - gout . Rheumatic fever - calcium . GC - pyrophosphate dihydrate (CPPD) . CTD (connective tissue diseases) - hydroxylapatite (HA) - RA . Spondyloarthropathies - systemic lupus erythematosus (SLE) - reactive . Sarcoidosis - psoriatic . - inflammatory bowel disease (IBD) Spondyloarthropathies - reactive - Reiters . - psoriatic Early RA - IBD - Reiters Non-inflammatory . Subacute bacterial endocarditis (SBE) . Trauma . Hemophilia Non-inflammatory . Avascular Necrosis . Hypertrophic osteoarthropathy . Internal derangement Chronic Monarthritis Chronic Polyarthritis Inflammatory Inflammatory . Chronic Infection . Bony erosions - fungal, - RA/Juvenile rheumatoid arthritis (JRA ) - tuberculosis (TB) - Crystal deposition . Rheumatoid arthritis (RA) - Infection (15%) - Erosive OA (rare) Non-inflammatory - Spondyloarthropathies -

Axial Spondyloarthritis
Central JSM Arthritis Review Article *Corresponding author Mali Jurkowski, Department of Internal Medicine, Temple University Hospital, 3401 North Broad Street, Axial Spondyloarthritis: Clinical Philadelphia, PA 19140, USA Submitted: 07 December 2020 Features, Classification, and Accepted: 31 January 2021 Published: 03 February 2021 ISSN: 2475-9155 Treatment Copyright Mali Jurkowski1*, Stephanie Jeong1 and Lawrence H Brent2 © 2021 Jurkowski M, et al. 1Department of Internal Medicine, Temple University Hospital, USA OPEN ACCESS 2Section of Rheumatology, Department of Medicine, Lewis Katz School of Medicine at Temple University, USA Keywords ABBREVIATIONS • Spondyloarthritis • Ankylosing spondylitis HLA-B27: human leukocyte antigen-B27; SpA: • HLA-B27 spondyloarthritis; AS: ankylosing spondylitis; nr-axSpA: non- • Classification criteria radiographic axial spondyloarthritis; ReA: reactive arthritis; PsA: psoriatic arthritis; IBD-SpA: disease associated spondyloarthritis; ASAS: Assessment of of rheumatoid arthritis [6]. AS affects men more than women SpondyloArthritis international Society; NSAIDs:inflammatory nonsteroidal bowel 79.6%, whereas nr-axSpA affects men and women equally 72.4% CASPAR: [7], independent of HLA-B27. This article will discuss the clinical for Psoriatic Arthritis; DMARDs: disease modifying anti- and diagnostic features of SpA, compare the classification criteria, rheumaticanti-inflammatory drugs; CD:drugs; Crohn’s disease; ClassificationUC: ulcerative Criteriacolitis; and provide updates regarding treatment options, including the ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; developmentCLINICAL FEATURESof biologics and targeted synthetic agents. TNFi: X-ray: plain radiography; MRI: magnetic resonance imaging; CT: computed Inflammatory back pain tomography;tumor US: necrosis ultrasonography; factor-α wb-MRI:inhibitors; ESSG: European Spondyloarthropathy Study Group; IL-17i: whole-body MRI; JAKi: PDE4i: Inflammatory back pain is the hallmark of SpA, present in 70- 80% of patients [8]. -

Clinical and Radiographic Features of Spondylitic Hip Disease J
Ann Rheum Dis: first published as 10.1136/ard.38.4.332 on 1 August 1979. Downloaded from Annals of the Rheumatic Diseases, 1979, 38, 332-336 Clinical and radiographic features of spondylitic hip disease J. S. MARKS AND K. HARDINGE From the Rheumatology Unit and the Centre for Hip Surgery, Wrightington Hospital, Wigan, Lancs SUMMARY The clinical and radiographic features of hip disease in 76 patients with definite anky- losing spondylitis have been studied. Symptomatic hip involvement occurred late in the course of the disease, with a mean delay after the onset of 12 years in males and 7 years in females. Patients with disease onset before the age of 20 developed hip symptoms at an earlier stage. Associated diseases included uveitis (13 %), colitis (4 %), and psoriasis (4 %). Bilateral concentric loss of hip joint space with a relatively undeformed femoral head was the commonest radiological change (61 %). Localised loss ofjoint space at the upper pole (16 %) was associated with femoral head destruction and a greater degree of osteophytosis, suggesting coincidental or secondary osteoarthrosis. Bony ankylosis of the hips (10%) was present only in women, and the absence of osteophytes, cysts, and bone lesions of the iliac crests and ischial rami suggests that it is a distinct radiographic manifestation of female ankylosing spondylitis. copyright. Ankylosing spondylitis characteristically affects Clinical details obtained from the medical records the sacroiliac joints and the spine, but peripheral included age at onset of disease, site(s) of initial joint involvement occurs in at least 50% of patients symptoms, age at initial hip symptoms, associated during the course of their disease (Polley and diseases, previous medical and surgical treatment, Slocumb, 1947; Wilkinson and Bywaters, 1958; and details of hip surgery during admission. -

Charcot Arthropathy: Differential Diagnosis of Inflammatory Arthritis
CHARCOT ARTHROPATHY: DIFFERENTIAL DIAGNOSIS OF INFLAMMATORY ARTHRITIS CAMILA DA SILVA CENDON DURAN (USP-SP, SÃO PAULO, SP, Brasil), CARLA BALEEIRO RODRIGUES SILVA (USP-SP, SÃO PAULO, SP, Brasil), CARLO SCOGNAMIGLIO RENNER ARAUJO (USP-SP, SÃO PAULO, SP, Brasil), LUCAS BRANDÃO ARAUJO DA SILVA (USP-SP, SÃO PAULO, SP, Brasil), LUCIANA PARENTE COSTA SEGURO (USP-SP, SÃO PAULO, SP, Brasil), LISSIANE KARINE NORONHA GUEDES (USP-SP, SÃO PAULO, SP, Brasil), ROSA MARIA RODRIGUES PEREIRA (USP-SP, SÃO PAULO, SP, Brasil), EDUARDO FERREIRA BORBA NETO (USP-SP, SÃO PAULO, SP, Brasil) BACKGROUND Charcot-Marie-Tooth Disease (CMT) is the most common hereditary neuropathy, with an estimated prevalence of 40 cases per 100,000 individuals. CMT type 2, the most common subtype, is an axonal disorder, usually beginning during the second or third decade of life. Clinical features include distal weakness, muscle atrophy, reduced sensitivity, decreased deep tendon reflexes and deformity of the foot. It may lead to the development of Charcot arthropathy, with increased osteoclastic activity and joint destruction. Ultrasonography can show effusion, synovitis, high-grade doppler activity and bone irregularities, findings that can lead to a misdiagnosis of inflammatory arthritis. CASE REPORT Male, 27-year-old, reported a history of pain and warm swelling of the left ankle and midfoot, worsening after exercise and improving with rest, that started nine years ago and persisted for one year. During this period, he self-medicated with non-steroidal anti-inflammatory drugs. He denied associated symptoms such as ocular inflammation, skin lesions, diarrhea, urethritis or fever. Ten months ago, he began to present similar symptoms in right ankle and midfoot, after a 7-year asymptomatic period. -

Hallux Valgus
MedicalContinuing Education Building Your FOOTWEAR PRACTICE Objectives 1) To be able to identify and evaluate the hallux abductovalgus deformity and associated pedal conditions 2) To know the current theory of etiology and pathomechanics of hallux valgus. 3) To know the results of recent Hallux Valgus empirical studies of the manage- ment of hallux valgus. Assessment and 4) To be aware of the role of conservative management, faulty footwear in the develop- ment of hallux valgus deformity. and the role of faulty footwear. 5) To know the pedorthic man- agement of hallux valgus and to be cognizant of the 10 rules for proper shoe fit. 6) To be familiar with all aspects of non-surgical management of hallux valgus and associated de- formities. Welcome to Podiatry Management’s CME Instructional program. Our journal has been approved as a sponsor of Continu- ing Medical Education by the Council on Podiatric Medical Education. You may enroll: 1) on a per issue basis (at $15 per topic) or 2) per year, for the special introductory rate of $99 (you save $51). You may submit the answer sheet, along with the other information requested, via mail, fax, or phone. In the near future, you may be able to submit via the Internet. If you correctly answer seventy (70%) of the questions correctly, you will receive a certificate attesting to your earned credits. You will also receive a record of any incorrectly answered questions. If you score less than 70%, you can retake the test at no additional cost. A list of states currently honoring CPME approved credits is listed on pg. -

Management of the First-Time Traumatic Anterior Shoulder Dislocation
CONCISE REVIEW CiSE Clinics in Shoulder and Elbow Clinics in Shoulder and Elbow Vol. 21, No. 3, September, 2018 https://doi.org/10.5397/cise.2018.21.3.169 Management of the First-time Traumatic Anterior Shoulder Dislocation Sung Il Wang Department of Orthopaedic Surgery, Chonbuk National University Medical School, Research Insitute for Endocrine Sciences and Research Insitute of Clinical Medicine of Chonbuk National University–Biomedical Research Insitute of Chonbuk National University Hospital, Jeonju, Korea Traumatic anterior dislocation of the shoulder is one of the most common directions of instability following a traumatic event. Although the incidence of shoulder dislocation is similar between young and elderly patients, most studies have traditionally focused on young pa- tients due to relatively high rates of recurrent dislocations in this population. However, shoulder dislocations in older patients also require careful evaluation and treatment selection because they can lead to persistent pain and disability due to rotator cuff tears and nerve injuries. This article provides an overview of the nature and pathology of acute primary anterior shoulder dislocation, widely accepted management modalities, and differences in treatment for young and elderly patients. (Clin Shoulder Elbow 2018;21(3):169-175) Key Words: Glenohumeral joint; Shoulder dislocation; Treatment Introduction the shoulder will invariably be damaged, rendering the joint un- stable. The glenohumeral joint has the greatest range of motion There are controversies over the best treatment for patients among all joints in the human body. To achieve increased with first-time anterior shoulder dislocation. Assessment of risk mobility, joint stability is sacrificed, making shoulder joint sus- factors for recurrence is essential when deciding on the treat- ceptible to dislocation. -
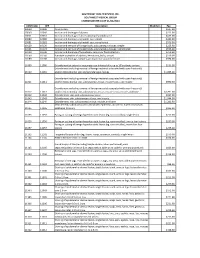
SOUTHWEST HEALTH SYSTEM, INC. SOUTHWEST MEDICAL GROUP CHARGEMASTER AS of 01/01/2021 CDM Code CPT Description Modifiers Fee 10040
SOUTHWEST HEALTH SYSTEM, INC. SOUTHWEST MEDICAL GROUP CHARGEMASTER AS OF 01/01/2021 CDM Code CPT Description Modifiers Fee 10040 10040 Acne surgery $147.00 10060 10060 Incision and drainage of abscess $212.00 10061 10061 Incision and drainage of abscess(multiple\complicated $422.00 10080 10080 Incision and drainage of pilonidal cyst; simple $340.00 10081 10081 Incision and drainage of pilonidal cyst; complicated $576.00 10120 10120 Incision and removal of foreign body, subcutaneous tissues; simple $225.00 10121 10121 Incision and removal of foreign body, subcutaneous tissues; complicated $513.00 10140 10140 Incision and drainage of hematoma, seroma or fluid collection $279.00 10160 10160 Puncture aspiration of abscess, hematoma, bulla, or cyst $179.00 10180 10180 Incision and drainage, complex, postoperative wound infection $594.00 11000 11000 Debridement of extensive eczematous or infected skin; up to 10% of body surface $133.00 Debridement including removal of foreign material associated with open fracture(s) 11010 11010 and/or dislocation(s); skin and subcutaneous tissues $1,085.00 Debridement including removal of foreign material associated with open fracture(s) 11011 11011 and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, and muscle $996.00 Debridement including removal of foreign material associated with open fracture(s) 11012 11012 and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, muscle, and bone $2,791.00 11042 11042 Debridement; skin, and subcutaneous tissue $197.00 11043 11043 Debridement; skin, -

Paleopathological Analysis of a Sub-Adult Allosaurus Fragilis (MOR
Paleopathological analysis of a sub-adult Allosaurus fragilis (MOR 693) from the Upper Jurassic Morrison Formation with multiple injuries and infections by Rebecca Rochelle Laws A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Earth Sciences Montana State University © Copyright by Rebecca Rochelle Laws (1996) Abstract: A sub-adult Allosaurus fragilis (Museum of the Rockies specimen number 693 or MOR 693; "Big Al") with nineteen abnormal skeletal elements was discovered in 1991 in the Upper Jurassic Morrison Formation in Big Horn County, Wyoming at what became known as the "Big Al" site. This site is 300 meters northeast of the Howe Quarry, excavated in 1934 by Barnum Brown. The opisthotonic position of the allosaur indicated that rigor mortis occurred before burial. Although the skeleton was found within a fluvially-deposited sandstone, the presence of mud chips in the sandstone matrix and virtual completeness of the skeleton showed that the skeleton was not transported very far, if at all. The specific goals of this study are to: 1) provide a complete description and analysis of the abnormal bones of the sub-adult, male, A. fragilis, 2) develop a better understanding of how the bones of this allosaur reacted to infection and trauma, and 3) contribute to the pathological bone database so that future comparative studies are possible, and the hypothesis that certain abnormalities characterize taxa may be evaluated. The morphology of each of the 19 abnormal bones is described and each disfigurement is classified as to its cause: 5 trauma-induced; 2 infection-induced; 1 trauma- and infection-induced; 4 trauma-induced or aberrant, specific origin unknown; 4 aberrant; and 3 aberrant, specific origin unknown. -

Subacromial Decompression in the Shoulder
Subacromial Decompression Geoffrey S. Van Thiel, Matthew T. Provencher, Shane J. Nho, and Anthony A. Romeo PROCEDURE 2 22 Indications P ITFALLS ■ Impingement symptoms refractory to at least • There are numerous possible 3 months of nonoperative management causes of shoulder pain that can ■ In conjunction with arthroscopic treatment of a mimic impingement symptoms. All potential causes should be rotator cuff tear thoroughly evaluated prior to ■ Relative indication: type II or III acromion with undertaking operative treatment clinical fi ndings of impingement of isolated impingement syndrome. Examination/Imaging Subacromial Decompression PHYSICAL EXAMINATION ■ Assess the patient for Controversies • Complete shoulder examination with range of • Subacromial decompression in motion and strength the treatment of rotator cuff • Tenderness with palpation over anterolateral pathology has been continually acromion and supraspinatus debated. Prospective studies • Classic Neer sign with anterolateral shoulder have suggested that there is no difference in outcomes with and pain on forward elevation above 90° when without subacromial the greater tuberosity impacts the anterior decompression. acromion (and made worse with internal rotation) • Subacromial decompression • Positive Hawkins sign: pain with internal rotation, performed in association with a forward elevation to 90°, and adduction, which superior labrum anterior- causes impingement against the coracoacromial posterior (SLAP) repair can potentially increase ligament postoperative stiffness. ■ The impingement test is positive if the patient experiences pain relief with a subacromial injection of lidocaine. ■ Be certain to evaluate for acromioclavicular (AC) joint pathology, and keep in mind that there are several causes of shoulder pain that can mimic impingement syndrome. P ITFALLS IMAGING • Ensure that an axillary lateral ■ Standard radiographs should be ordered, view is obtained to rule out an os acromiale.