Topdown and Bottomup Influences on Demographic Rates of Antarctic Fur
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploitation of Fur Seals and Sea Lions from Australian, New Zealand and Adjacent Subantarctic Islands During the Eighteenth, Nineteenth and Twentieth Centuries
Exploitation of fur seals and sea lions from Australian, New Zealand and adjacent subantarctic islands during the eighteenth, nineteenth and twentieth centuries John K. Ling P.O. Box 271, Clare, South Australia 5459 ABSTRACT Details of skin cargoes of fur seal Arctocephalus spp. and sea lion Neophoca cinerea and Phocarctos hookeri, originating from southern Australia, New Zealand and the adjacent subantarctic islands in the lath, 19th and 20th centuries have been collated from several secondary historicsl sources. These sources quoted quantities of skins in terms of actual tallied numbers, as untallied "cargoes" or as casks, sacks or bundles. Untallied cargoes were Downloaded from http://meridian.allenpress.com/australian-zoologist/article-pdf/31/2/323/1473420/az_1999_036.pdf by guest on 30 September 2021 converted into numbers by averaging tallied cargoes; and casks, sacks and bundles were arbitrarily deemed to contain 40, 20 and 5 skins respectively. Annual and total yields of skins are presented for ten separate areas in the region: Bass Strait, King Island, Kangaroo Island. Western Australia, New Zealand, Bounty Islands, Auckland Islands, Antipodes Islands. Campbell Island and Macquarie island. At least 1 367 000 fur seal skins were harvested between 1792 and 1948149 in the whole of the Australasian region. More than 1309 000 skins - 96% of the total - were taken up to 1830. Records indicate that only about 4 100 Neophoca and 5 800 Phocarctos were obtained from their respective areas. These figures must be regarded as minimal, as it is likely thatmany cargoes were obtained by English, American and French vessels and shipped directly to European or Asian markets. -

56. Otariidae and Phocidae
FAUNA of AUSTRALIA 56. OTARIIDAE AND PHOCIDAE JUDITH E. KING 1 Australian Sea-lion–Neophoca cinerea [G. Ross] Southern Elephant Seal–Mirounga leonina [G. Ross] Ross Seal, with pup–Ommatophoca rossii [J. Libke] Australian Sea-lion–Neophoca cinerea [G. Ross] Weddell Seal–Leptonychotes weddellii [P. Shaughnessy] New Zealand Fur-seal–Arctocephalus forsteri [G. Ross] Crab-eater Seal–Lobodon carcinophagus [P. Shaughnessy] 56. OTARIIDAE AND PHOCIDAE DEFINITION AND GENERAL DESCRIPTION Pinnipeds are aquatic carnivores. They differ from other mammals in their streamlined shape, reduction of pinnae and adaptation of both fore and hind feet to form flippers. In the skull, the orbits are enlarged, the lacrimal bones are absent or indistinct and there are never more than three upper and two lower incisors. The cheek teeth are nearly homodont and some conditions of the ear that are very distinctive (Repenning 1972). Both superfamilies of pinnipeds, Phocoidea and Otarioidea, are represented in Australian waters by a number of species (Table 56.1). The various superfamilies and families may be distinguished by important and/or easily observed characters (Table 56.2). King (1983b) provided more detailed lists and references. These and other differences between the above two groups are not regarded as being of great significance, especially as an undoubted fur seal (Australian Fur-seal Arctocephalus pusillus) is as big as some of the sea lions and has some characters of the skull, teeth and behaviour which are rather more like sea lions (Repenning, Peterson & Hubbs 1971; Warneke & Shaughnessy 1985). The Phocoidea includes the single Family Phocidae – the ‘true seals’, distinguished from the Otariidae by the absence of a pinna and by the position of the hind flippers (Fig. -

Hunting and Social Behaviour of Leopard Seals (Hydrurga Leptonyx) at Seal Island, South Shetland Islands, Antarctica
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Publications, Agencies and Staff of the U.S. Department of Commerce U.S. Department of Commerce 1999 Hunting and social behaviour of leopard seals (Hydrurga leptonyx) at Seal Island, South Shetland Islands, Antarctica Lisa M. Hiruki National Marine Mammal Laboratory, Alaska Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, [email protected] Michael K. Schwartz National Marine Mammal Laboratory, Alaska Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration Peter L. Boveng National Marine Mammal Laboratory, Alaska Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration Follow this and additional works at: https://digitalcommons.unl.edu/usdeptcommercepub Part of the Environmental Sciences Commons Hiruki, Lisa M.; Schwartz, Michael K.; and Boveng, Peter L., "Hunting and social behaviour of leopard seals (Hydrurga leptonyx) at Seal Island, South Shetland Islands, Antarctica" (1999). Publications, Agencies and Staff of the U.S. Department of Commerce. 151. https://digitalcommons.unl.edu/usdeptcommercepub/151 This Article is brought to you for free and open access by the U.S. Department of Commerce at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications, Agencies and Staff of the U.S. Department of Commerce by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. J. Zool., Lond. (1999) 249, 97±109 # 1999 The Zoological Society of London Printed in the United Kingdom Hunting and social behaviour of leopard seals (Hydrurga leptonyx) at Seal Island, South Shetland Islands, Antarctica Lisa M. Hiruki*, Michael K. Schwartz{ and Peter L. -
Global Patterns in Marine Mammal Distributions
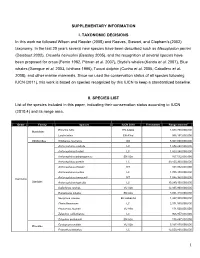
SUPPLEMENTARY INFORMATION I. TAXONOMIC DECISIONS In this work we followed Wilson and Reeder (2005) and Reeves, Stewart, and Clapham’s (2002) taxonomy. In the last 20 years several new species have been described such as Mesoplodon perrini (Dalebout 2002), Orcaella heinsohni (Beasley 2005), and the recognition of several species have been proposed for orcas (Perrin 1982, Pitman et al. 2007), Bryde's whales (Kanda et al. 2007), Blue whales (Garrigue et al. 2003, Ichihara 1996), Tucuxi dolphin (Cunha et al. 2005, Caballero et al. 2008), and other marine mammals. Since we used the conservation status of all species following IUCN (2011), this work is based on species recognized by this IUCN to keep a standardized baseline. II. SPECIES LIST List of the species included in this paper, indicating their conservation status according to IUCN (2010.4) and its range area. Order Family Species IUCN 2010 Freshwater Range area km2 Enhydra lutris EN A2abe 1,084,750,000,000 Mustelidae Lontra felina EN A3cd 996,197,000,000 Odobenidae Odobenus rosmarus DD 5,367,060,000,000 Arctocephalus australis LC 1,674,290,000,000 Arctocephalus forsteri LC 1,823,240,000,000 Arctocephalus galapagoensis EN A2a 167,512,000,000 Arctocephalus gazella LC 39,155,300,000,000 Arctocephalus philippii NT 163,932,000,000 Arctocephalus pusillus LC 1,705,430,000,000 Arctocephalus townsendi NT 1,045,950,000,000 Carnivora Otariidae Arctocephalus tropicalis LC 39,249,100,000,000 Callorhinus ursinus VU A2b 12,935,900,000,000 Eumetopias jubatus EN A2a 3,051,310,000,000 Neophoca cinerea -

The Antarctic Ross Seal, and Convergences with Other Mammals
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Servicio de Difusión de la Creación Intelectual Evolutionary biology Sensory anatomy of the most aquatic of rsbl.royalsocietypublishing.org carnivorans: the Antarctic Ross seal, and convergences with other mammals Research Cleopatra Mara Loza1, Ashley E. Latimer2,†, Marcelo R. Sa´nchez-Villagra2 and Alfredo A. Carlini1 Cite this article: Loza CM, Latimer AE, 1 Sa´nchez-Villagra MR, Carlini AA. 2017 Sensory Divisio´n Paleontologı´a de Vertebrados, Museo de La Plata, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata, Argentina. CONICET, La Plata, Argentina anatomy of the most aquatic of carnivorans: 2Pala¨ontologisches Institut und Museum der Universita¨tZu¨rich, Karl-Schmid Strasse 4, 8006 Zu¨rich, Switzerland the Antarctic Ross seal, and convergences with MRS-V, 0000-0001-7587-3648 other mammals. Biol. Lett. 13: 20170489. http://dx.doi.org/10.1098/rsbl.2017.0489 Transitions to and from aquatic life involve transformations in sensory sys- tems. The Ross seal, Ommatophoca rossii, offers the chance to investigate the cranio-sensory anatomy in the most aquatic of all seals. The use of non-invasive computed tomography on specimens of this rare animal Received: 1 August 2017 reveals, relative to other species of phocids, a reduction in the diameters Accepted: 12 September 2017 of the semicircular canals and the parafloccular volume. These features are independent of size effects. These transformations parallel those recorded in cetaceans, but these do not extend to other morphological features such as the reduction in eye muscles and the length of the neck, emphasizing the independence of some traits in convergent evolution to aquatic life. -

Inter-Year Variation in Pup Production of Caspian Seals Pusa Caspica 2005–2012 Determined from Aerial Surveys
Vol. 28: 209–223, 2015 ENDANGERED SPECIES RESEARCH Published online October 7 doi: 10.3354/esr00689 Endang Species Res OPEN ACCESS Inter-year variation in pup production of Caspian seals Pusa caspica 2005–2012 determined from aerial surveys Lilia Dmitrieva1,*, Tero Härkönen2, Mirgaliy Baimukanov3, Anders Bignert2, Ivar Jüssi4, Mart Jüssi4, Yesbol Kasimbekov3, Mikhail Verevkin5, Vadim Vysotskiy6, Susan Wilson7, Simon J. Goodman1,* 1School of Biology, University of Leeds, Leeds LS2 9JT, UK 2Swedish Museum of Natural History, Box 50007, Stockholm 10405, Sweden 3Institute of Hydrobiology & Ecology, Karasaysky Raion, Almaty 040916, Kazakhstan 4Estonian Fund for Nature, PO Box 245, Tartu 50002, Estonia 5St. Petersburg State University, Universitetskaya nab.7/9, St. Petersburg 199034, Russia 6Zoological Institute, RAS, Universitetskaja nab. 1, St. Petersburg 199034, Russia 7Tara Seal Research, Killyleagh, Co. Down BT30 9QN, UK ABSTRACT: Assessing species abundance and reproductive output is crucial for evaluations of population dynamics, conservation status and the development of management objectives. The Caspian seal Pusa caspica is a key predator in the Caspian Sea ecosystem and is listed as Endan- gered by the IUCN. Here we report on fixed-wing aerial strip transect surveys of the breeding population on the Caspian Sea winter ice field carried out in February, 2005−2012. Potential detection biases were estimated by applying a Petersen mark–recapture estimator to the counts from double photographic observations. We also tested for effects of weather conditions on count results, and for correlations between pup production and ice conditions and net primary produc- tivity (npp). Fluctuations in pup production estimates were observed among years, ranging from 8200 pups (95% CI: 7130−9342) in 2010 to 34 000 (95% CI: 31 275−36 814) in 2005. -

PINNIPEDS (Seals, Sea Lions and Fur Seals) of NEW ZEALAND
PINNIPEDS (seals, sea lions and fur seals) OF NEW ZEALAND Subantarctic Fur Seal New Zealand Fur Seal Arctocephalis tropicalis Arctocephalus forsteri New Zealand Sea Lion Phocarctos hookeri Leopard Seal Hydrurga leptonyx Southern Elephant Seal Mirounga leonina © Emma Scheltema, 2015 PINNIPEDS OF NEW ZEALAND What are Pinnipeds? Pinnipeds are fin-footed (pinna = fin and pedis = foot) carnivorous marine mammals, commonly known as seals. They make up the largest group of native mammals that breed on land, in New Zealand. There are two living families of pinnipeds- theOtariidae (eared seals- which are the fur seals and sea lions) and Phocidae (earless or true seals). Otariidae can be identified by theirexternal ears (Phocidae have none)and their mobile hind flippers, giving them the ability to move around quickly on land. In contrast Phocidae have trouble moving on land as they cannot turn their hind flippers forward. There are eight pinniped species that are native to the New Zealand region. The five species highlighted in this coloring sheet are the species that are regular or occasional visitors to our mainland shores and subantarctic islands. OTARIIDAE (eared seals) (1) New Zealand Fur Seal (Kekeno) (2) Subantarctic Fur Seal (3) New Zealand Sea Lion (Whakahao) Native, Protected Vagrant Endemic, Protected The New Zealand Fur Seal is the most common Characteristics: very similar to The NZ Sealion is one of the rarest species of sea seal spotted on NZ coastlines NZ Fur Seal, dark grey-brown lion in the world. fur on back, with a yellow-cream Characteristics: dark grey-brown fur, with chest. Males have a head crest Characteristics: Males are dark brown to black distinguishing pointed snout, and under-eyes are which is visible when agitated. -

Marine Mammal Taxonomy
Marine Mammal Taxonomy Kingdom: Animalia (Animals) Phylum: Chordata (Animals with notochords) Subphylum: Vertebrata (Vertebrates) Class: Mammalia (Mammals) Order: Cetacea (Cetaceans) Suborder: Mysticeti (Baleen Whales) Family: Balaenidae (Right Whales) Balaena mysticetus Bowhead whale Eubalaena australis Southern right whale Eubalaena glacialis North Atlantic right whale Eubalaena japonica North Pacific right whale Family: Neobalaenidae (Pygmy Right Whale) Caperea marginata Pygmy right whale Family: Eschrichtiidae (Grey Whale) Eschrichtius robustus Grey whale Family: Balaenopteridae (Rorquals) Balaenoptera acutorostrata Minke whale Balaenoptera bonaerensis Arctic Minke whale Balaenoptera borealis Sei whale Balaenoptera edeni Byrde’s whale Balaenoptera musculus Blue whale Balaenoptera physalus Fin whale Megaptera novaeangliae Humpback whale Order: Cetacea (Cetaceans) Suborder: Odontoceti (Toothed Whales) Family: Physeteridae (Sperm Whale) Physeter macrocephalus Sperm whale Family: Kogiidae (Pygmy and Dwarf Sperm Whales) Kogia breviceps Pygmy sperm whale Kogia sima Dwarf sperm whale DOLPHIN R ESEARCH C ENTER , 58901 Overseas Hwy, Grassy Key, FL 33050 (305) 289 -1121 www.dolphins.org Family: Platanistidae (South Asian River Dolphin) Platanista gangetica gangetica South Asian river dolphin (also known as Ganges and Indus river dolphins) Family: Iniidae (Amazon River Dolphin) Inia geoffrensis Amazon river dolphin (boto) Family: Lipotidae (Chinese River Dolphin) Lipotes vexillifer Chinese river dolphin (baiji) Family: Pontoporiidae (Franciscana) -

THE PINNIPEDS of the CALIFORNIA CURRENT California
ANTONELIS AND FISCUS: PINNIPEDS OF THE CALIFORNIA CURRENT CalCOFI Rep., Vol. XXI, 1980 THE PINNIPEDS OF THE CALIFORNIA CURRENT GEORGE A. ANTONELIS. JR. AND CLIFFORD H. FISCUS Marine Mammal Division Northwest and Alaska Fisheries Center National Marine Fisheries Service National Oceanic and Atmospheric Administration 7600 Sand Point Way, N.E. Seattle, WA 981 15 ABSTRACT 10s pequenos peces en 10s cardumenes y peces ana- There are six species of pinnipeds-California sea dromos. Los dos focidos, otra vez con ciertas excep- lion, Zalophus californianus; northern sea lion, Eume- ciones, predan especies diferentes. Aparentemente, el topias jubatus; northern fur seal, Callorhinus ursinus; elefante marino se alimenta en aguas mas profundas que Guadalupe fur seal, Arctocephalus townsendi; harbor la foca peluda, alimentindose de especies demersales seal, Phoca uitulina richardsi; and northern elephant y benticas, y la foca peluda se alimenta de especiesdemer- seal, Mirounga angustirostris-that inhabit the study sales costeras y neriticas, entrando ocasionalmente en rios area of the California Cooperative Oceanic Fisheries y aguas estuarinas haciendopresa de 10s peces anadromos Investigations (CalCOFI). y otros pequeiios peces que entran regularmente en estas The numbers of animals in each population are given; aguas. the size, distribution, and seasonal movements are de- scribed. The known prey species of the pinnipeds are INTRODUCTION listed for each species. The otariids, with certain excep- The California Current, its components, and the Cali- tions, consume the same kinds of prey, although in slight- fornia Cooperative Oceanic Fisheries Investigations ly different amounts. In general they feed most commonly (CalCOFI) station plan have been described many times on the smaller schooling fishes and squids of the epi- in the past and are well known (Kramer et al. -

Issues Paper for the Australian Sea Lion(Neophoca Cinerea)
Issues Paper for the Australian Sea Lion (Neophoca cinerea) 2013 The recovery plan linked to this issues paper is obtainable from: www.environment.gov.au/coasts/species/seals/index.html © Commonwealth of Australia 2013 This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any use as permitted under the Copyright Act 1968, all other rights are reserved. Requests and enquiries concerning reproduction and rights should be addressed to Department of Sustainability, Environment, Water, Population and Communities, Public Affairs, GPO Box 787 Canberra ACT 2601 or email [email protected]. Disclaimer While reasonable efforts have been made to ensure that the contents of this publication are factually correct, the Commonwealth does not accept responsibility for the accuracy or completeness of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the contents of this publication. Images credits Front cover left to right: entangled Australian sea lion, close up image of Australian sea lion, colony of Australian sea lions, Australian sea lion on the water’s surface – Derek Hamer, Australian sea lion underwater – David Muirhead Back cover left to right: Australian sea lion on a rocky shore, close up image of Australian sea lion, Australian sea lion on the water’s surface – Derek Hamer 2 | -

The Role of Pinnipeds in the Eeosystem Dr
The Role of Pinnipeds in the EEosystem Dr. Andrew W. Trites, Marine Mammal Research Unit, Fisheries Centre, University of British Columbia, Vancouver, British Columbia Abstract The proximate role played by seals and sea lions is obvious: they are predators and consumers of fish and invertebrates. Less intuitive is their ultimate role (dynamic and structural) within the ecosystem. The limited information available suggests that some pinnipeds perform a dynamic role by transferring nutrients and energy, or by regulating the abundance of other species. Others may play a structural role by influencing the physical complexity of their environment; or they may synthesize the marine environment and serve as indicators of ecosystem change. Field observations suggest the ultimate role thatpinnipedsfill is species specific and a function of the type of habitat and ecosystem they occupy. Their functional and structural roles appear to be most evident in simple short-chained food webs, and are least obvious and tractable in complex long-chained food webs due perhaps to high variability in the recruitment offish or nonlinear interactions and responses of predators and prey. The impact of historic removals of whales, sea otters and seals are consistent with these observations. Many of these removals produced unexpected changes in other components of the ecosystem. Better insights into the role that pinnipeds play and the effect of removing them will come as better data on diets and predator-prey functional responses are included in ecosystem models. Introduction What role do pinnipeds play in the ecosystem? Are they at all-important to the ecosystem or is the ecosystem more important to them? These questions are not easily answered, but are important to those concerned with fisheries, marine mammals, and the health of the marine environment. -

Monk Seal Action Plan English Version 2005
Convention on the Conservation of Migratory Species of Wild Animals Secretariat provided by the United Nations Environment Programme THIRTEENTH MEETING OF THE CMS SCIENTIFIC COUNCIL Nairobi, Kenya, 16-18 November 2005 CMS/ScC.13/Inf.3 ACTION PLAN FOR THE RECOVERY OF THE MEDITERRANEAN MONK SEAL IN THE EASTERN ATLANTIC Under this cover is reproduced the Action Plan for the Recovery of the Mediterranean Monk Seal in the Eastern Atlantic, elaborated within the Concerted Action for the species. The document is reproduced as received from the Government of Spain, lead country in the development of the Concerted Action, without editing. 2 ACTIOON PPLAANN FOORR TTHHEE RREECOVERRYY OOFF THE MMEDDIITTEERRRAANEEAN MOONNKK SSEAALL IIN TTHHEE EEASSTERRNN ATLAANNTIC CONVENTION O N THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS (BONN CONVENTION) 1 ACTION PLAN FOR THE RECOVERY OF THE MEDITERRANEAN MONK SEAL (Monachus monachus ) IN THE EASTERN ATLANTIC Prepared by the WORKING GROUP OF THE MEDITERRAEAN MONK SEAL IN THE EASTERN ATLANTIC For the CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS (BONN CONVENTION) 2005 2 TABLE OF CONTENTS SUMMARY .....................................................................................................................................................4 MEMBERS OF THE ATLANTIC MONK SEAL WORKING GROUP...............................................5 ACKNOWLEDGEMENTS ..........................................................................................................................6 LIST