Section 1: Anatomy of the Sensorimotor System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -

(Mpfc-Lpmc) Affects Subjective Beauty but Not Ugliness
ORIGINAL RESEARCH published: 08 December 2015 doi: 10.3389/fnhum.2015.00654 Transcranial Direct Current Stimulation Over the Medial Prefrontal Cortex and Left Primary Motor Cortex (mPFC-lPMC) Affects Subjective Beauty but not Ugliness Koyo Nakamura1 and Hideaki Kawabata2* 1 Graduate School of Human Relations, Keio University, Tokyo, Japan, 2 Department of Psychology, Keio University, Tokyo, Japan Neuroaesthetics has been searching for the neural bases of the subjective experience of beauty. It has been demonstrated that neural activities in the medial prefrontal cortex (mPFC) and the left primary motor cortex (lPMC) correlate with the subjective experience of beauty. Although beauty and ugliness seem to be semantically and conceptually opposite, it is still unknown whether these two evaluations represent extreme opposites in unitary or bivariate dimensions. In this study, we applied transcranial direct current stimulation (tDCS) to examine whether non-invasive brain stimulation modulates two types of esthetic evaluation; evaluating beauty and ugliness. Participants rated the Edited by: subjective beauty and ugliness of abstract paintings before and after the application of Edward A. Vessel, tDCS. Application of cathodal tDCS over the mPFC with anode electrode over the lPMC, New York University, USA which induced temporal inhibition of neural excitability of the mPFC, led to a decrease Reviewed by: Zaira Cattaneo, in beauty ratings but not ugliness ratings. There were no changes in ratings of both University of Milano-Bicocca, Italy beauty and ugliness when applying anodal tDCS or sham stimulation over the mPFC. Andrea Antal, Results from our experiment indicate that the mPFC and the lPMC have a causal role University Medical Center Goettingen, Germany in generating the subjective experience of beauty, with beauty and ugliness evaluations Gerald Cupchik, constituting two distinct dimensions. -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -

Prefrontal and Posterior Parietal Contributions to the Perceptual Awareness of Touch M
www.nature.com/scientificreports OPEN Prefrontal and posterior parietal contributions to the perceptual awareness of touch M. Rullmann1,2,5, S. Preusser1,5 & B. Pleger1,3,4* Which brain regions contribute to the perceptual awareness of touch remains largely unclear. We collected structural magnetic resonance imaging scans and neurological examination reports of 70 patients with brain injuries or stroke in S1 extending into adjacent parietal, temporal or pre-/frontal regions. We applied voxel-based lesion-symptom mapping to identify brain areas that overlap with an impaired touch perception (i.e., hypoesthesia). As expected, patients with hypoesthesia (n = 43) presented lesions in all Brodmann areas in S1 on postcentral gyrus (BA 1, 2, 3a, 3b). At the anterior border to BA 3b, we additionally identifed motor area BA 4p in association with hypoesthesia, as well as further ventrally the ventral premotor cortex (BA 6, BA 44), assumed to be involved in whole-body perception. At the posterior border to S1, we found hypoesthesia associated efects in attention-related areas such as the inferior parietal lobe and intraparietal sulcus. Downstream to S1, we replicated previously reported lesion-hypoesthesia associations in the parietal operculum and insular cortex (i.e., ventral pathway of somatosensory processing). The present fndings extend this pathway from S1 to the insular cortex by prefrontal and posterior parietal areas involved in multisensory integration and attention processes. Te primary somatosensory cortex (S1) in monkeys can be divided into four Brodmann areas: (BA) 1, 2, 3a, and 3b. Each BA consists of a somatotopically organized map that subserves distinct somatosensory functions1–3. -

3D Histological Reconstruction of Fiber Tracts and Direct Comparison with Diffusion Tensor MRI Tractography
3D Histological Reconstruction of Fiber Tracts and Direct Comparison with Diffusion Tensor MRI Tractography Julien Dauguet1, Sharon Peled2, Vladimir Berezovskii3, Thierry Delzescaux4, Simon K. Warfield1, Richard Born3, and Carl-Fredrik Westin5 1 Computational Radiology Laboratory, Children’s Hospital, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA 2 Harvard Center for Neurodegeneration and Repair, Boston, USA 3 Department of Neurobiology, Harvard Medical School, Boston, USA 4 Service Hospitalier Fr´ed´eric Joliot, CEA, Orsay, France 5 Laboratory of Mathematics in Imaging, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA Abstract. A classical neural tract tracer, WGA-HRP, was injected at multiple sites within the brain of a macaque monkey. Histological sections of the labeled fiber tracts were reconstructed in 3D, and the fibers were segmented and registered with the anatomical post-mortem MRI from the same animal. Fiber tracing along the same pathways was performed on the DTI data using a classical diffusion tracing technique. The fibers derived from the DTI were compared with those segmented from the histology in order to evaluate the performance of DTI fiber tracing. While there was generally good agreement between the two methods, our results reveal certain limitations of DTI tractography, particularly at regions of fiber tract crossing or bifurcation. 1 Introduction Tracing neural connections lies at the heart of neuroanatomy, and has profound implications for the study of neural function and for the study of developmental and adult plasticity of the nervous system. It is also important for the study of neurodegenerative diseases, and for the planning of neurosurgical procedures, such as tumor ablation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Basal Ganglia Anatomy, Physiology, and Function Ns201c
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN Analagous rodent basal ganglia nuclei Gross anatomy of the striatum: gateway to the basal ganglia rodent Dorsomedial striatum: -Inputs predominantly from mPFC, thalamus, VTA Dorsolateral striatum: -Inputs from sensorimotor cortex, thalamus, SNc Ventral striatum: Striatal subregions: Dorsomedial (caudate) -Inputs from vPFC, hippocampus, amygdala, Dorsolateral (putamen) thalamus, VTA Ventral (nucleus accumbens) Gross anatomy of the striatum: patch and matrix compartments Patch/Striosome: -substance P -mu-opioid receptor Matrix: -ChAT and AChE -somatostatin Microanatomy of the striatum: cell types Projection neurons: MSN: medium spiny neuron (GABA) •striatonigral projecting – ‘direct pathway’ •striatopallidal projecting – ‘indirect pathway’ Interneurons: FS: fast-spiking interneuron (GABA) LTS: low-threshold spiking interneuron (GABA) LA: large aspiny neuron (ACh) 30 um Cellular properties of striatal neurons Microanatomy of the striatum: striatal microcircuits • Feedforward inhibition (mediated by fast-spiking interneurons) • Lateral feedback inhibition (mediated by MSN collaterals) Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN The simplified ‘classical’ model of basal ganglia circuit function • Information encoded as firing rate • Basal ganglia circuit is linear and unidirectional • Dopamine exerts opposing effects on direct and indirect pathway MSNs Basal ganglia motor circuit: direct pathway Motor Cortex Premotor Cortex Glutamate Striatum GPe GPi/SNr Dopamine + GABA Motor Thalamus SNc STN Direct pathway MSNs express: D1, M4 receptors, Sub. -

Chapter 8 Nervous System
Chapter 8 Nervous System I. Functions A. Sensory Input – stimuli interpreted as touch, taste, temperature, smell, sound, blood pressure, and body position. B. Integration – CNS processes sensory input and initiates responses categorizing into immediate response, memory, or ignore C. Homeostasis – maintains through sensory input and integration by stimulating or inhibiting other systems D. Mental Activity – consciousness, memory, thinking E. Control of Muscles & Glands – controls skeletal muscle and helps control/regulate smooth muscle, cardiac muscle, and glands II. Divisions of the Nervous system – 2 anatomical/main divisions A. CNS (Central Nervous System) – consists of the brain and spinal cord B. PNS (Peripheral Nervous System) – consists of ganglia and nerves outside the brain and spinal cord – has 2 subdivisions 1. Sensory Division (Afferent) – conducts action potentials from PNS toward the CNS (by way of the sensory neurons) for evaluation 2. Motor Division (Efferent) – conducts action potentials from CNS toward the PNS (by way of the motor neurons) creating a response from an effector organ – has 2 subdivisions a. Somatic Motor System – controls skeletal muscle only b. Autonomic System – controls/effects smooth muscle, cardiac muscle, and glands – 2 branches • Sympathetic – accelerator “fight or flight” • Parasympathetic – brake “resting and digesting” * 4 Types of Effector Organs: skeletal muscle, smooth muscle, cardiac muscle, and glands. III. Cells of the Nervous System A. Neurons – receive stimuli and transmit action potentials -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -
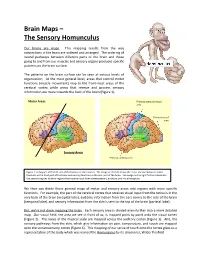
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Functional Role of the Supplementary and Pre-Supplementary Motor Areas
REVIEWS Functional role of the supplementary and pre-supplementary motor areas Parashkev Nachev*‡, Christopher Kennard‡ and Masud Husain* Abstract | The supplementary motor complex consists of the supplementary motor area, the supplementary eye field and the pre-supplementary motor area. In recent years, these areas have come under increasing scrutiny from cognitive neuroscientists, motor physiologists and clinicians because they seem to be crucial for linking cognition to action. However, theories regarding their function vary widely. This Review brings together the data regarding the supplementary motor regions, highlighting outstanding issues and providing new perspectives for understanding their functions. Ever since they were first identified, the regions that which we hope will stimulate the development of comprise the supplementary motor complex (SMC) have conceptual frameworks for a better understanding remained, in large part, a mystery. Most investigators now of this region. appreciate that these brain regions are far from ‘supple- mentary’ to requirements1–7. Without them, there are Anatomy and connections profound alterations in behaviour. For example, lesions to The supplementary motor area (SMA) and pre- these areas in humans can lead to alien-limb syndrome, supplementary motor area (pre-SMA) are, in humans, with patients demonstrating involuntary actions such as located on the medial aspect of the brain: in the dorso- grasping nearby objects — even other people — without medial frontal cortex3,14, anterior to the leg representa- ever intending to do so8,9. Some individuals demonstrate tion of the primary motor cortex (FIG. 1). Both areas lie utilization behaviour: unable to resist the impulse to use in the superior frontal gyrus and constitute the medial an object that has been placed within their reach, even part of Brodmann’s area 6c (later divided into two areas: when the object is not needed10.