The Retinas of Mantis Shrimps from Low-Light Environments (Crustacea; Stomatopoda; Gonodactylidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Learning in Stomatopod Crustaceans
International Journal of Comparative Psychology, 2006, 19 , 297-317. Copyright 2006 by the International Society for Comparative Psychology Learning in Stomatopod Crustaceans Thomas W. Cronin University of Maryland Baltimore County, U.S.A. Roy L. Caldwell University of California, Berkeley, U.S.A. Justin Marshall University of Queensland, Australia The stomatopod crustaceans, or mantis shrimps, are marine predators that stalk or ambush prey and that have complex intraspecific communication behavior. Their active lifestyles, means of predation, and intricate displays all require unusual flexibility in interacting with the world around them, imply- ing a well-developed ability to learn. Stomatopods have highly evolved sensory systems, including some of the most specialized visual systems known for any animal group. Some species have been demonstrated to learn how to recognize and use novel, artificial burrows, while others are known to learn how to identify novel prey species and handle them for effective predation. Stomatopods learn the identities of individual competitors and mates, using both chemical and visual cues. Furthermore, stomatopods can be trained for psychophysical examination of their sensory abilities, including dem- onstration of color and polarization vision. These flexible and intelligent invertebrates continue to be attractive subjects for basic research on learning in animals with relatively simple nervous systems. Among the most captivating of all arthropods are the stomatopod crusta- ceans, or mantis shrimps. These marine creatures, unfamiliar to most biologists, are abundant members of shallow marine ecosystems, where they are often the dominant invertebrate predators. Their common name refers to their method of capturing prey using a folded, anterior raptorial appendage that looks superficially like the foreleg of a praying mantis. -

Linkage Mechanics and Power Amplification of the Mantis Shrimp's
3677 The Journal of Experimental Biology 210, 3677-3688 Published by The Company of Biologists 2007 doi:10.1242/jeb.006486 Linkage mechanics and power amplification of the mantis shrimp’s strike S. N. Patek1,*, B. N. Nowroozi2, J. E. Baio1, R. L. Caldwell1 and A. P. Summers2 1Department of Integrative Biology, University of California, Berkeley, CA 94720-3140, USA and 2Ecology and Evolutionary Biology, University of California–Irvine, Irvine, CA 92697-2525, USA *Author for correspondence (e-mail: [email protected]) Accepted 6 August 2007 Summary Mantis shrimp (Stomatopoda) generate extremely rapid transmission is lower than predicted by the four-bar model. and forceful predatory strikes through a suite of structural The results of the morphological, kinematic and modifications of their raptorial appendages. Here we mechanical analyses suggest a multi-faceted mechanical examine the key morphological and kinematic components system that integrates latches, linkages and lever arms and of the raptorial strike that amplify the power output of the is powered by multiple sites of cuticular energy storage. underlying muscle contractions. Morphological analyses of Through reorganization of joint architecture and joint mechanics are integrated with CT scans of asymmetric distribution of mineralized cuticle, the mantis mineralization patterns and kinematic analyses toward the shrimp’s raptorial appendage offers a remarkable example goal of understanding the mechanical basis of linkage of how structural and mechanical modifications can yield dynamics and strike performance. We test whether a four- power amplification sufficient to produce speeds and forces bar linkage mechanism amplifies rotation in this system at the outer known limits of biological systems. -

Stomatopod Interrelationships: Preliminary Results Based on Analysis of Three Molecular Loci
Arthropod Systematics & Phylogeny 91 67 (1) 91 – 98 © Museum für Tierkunde Dresden, eISSN 1864-8312, 17.6.2009 Stomatopod Interrelationships: Preliminary Results Based on Analysis of three Molecular Loci SHANE T. AHYONG 1 & SIMON N. JARMAN 2 1 Marine Biodiversity and Biodescurity, National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington, New Zealand [[email protected]] 2 Australian Antarctic Division, 203 Channel Highway, Kingston, Tasmania 7050, Australia [[email protected]] Received 16.iii.2009, accepted 15.iv.2009. Published online at www.arthropod-systematics.de on 17.vi.2009. > Abstract The mantis shrimps (Stomatopoda) are quintessential marine predators. The combination of powerful raptorial appendages and remarkably developed sensory systems place the stomatopods among the most effi cient invertebrate predators. High level phylogenetic analyses have been so far based on morphology. Crown-group Unipeltata appear to have diverged in two broad directions from the outset – one towards highly effi cient ‘spearing’ with multispinous dactyli on the raptorial claws (dominated by Lysiosquilloidea and Squilloidea), and the other towards ‘smashing’ (Gonodactyloidea). In a preliminary molecular study of stomatopod interrelationships, we assemble molecular data for mitochondrial 12S and 16S regions, combined with new sequences from the 16S and two regions of the nuclear 28S rDNA to compare with morphological hypotheses. Nineteen species representing 9 of 17 extant families and 3 of 7 superfamilies were analysed. The molecular data refl ect the overall patterns derived from morphology, especially in a monophyletic Squilloidea, a monophyletic Lysiosquilloidea and a monophyletic clade of gonodactyloid smashers. Molecular analyses, however, suggest the novel possibility that Hemisquillidae and possibly Pseudosquillidae, rather than being basal or near basal in Gonodactyloidea, may be basal overall to the extant stomatopods. -

MANTIS SHRIMPSSHRIMPS Fast, Flexible, Fearless - Scurrying and Scooting Among the Coral Rubble Or Suddenly Exploding from Their Burrows in the Muck
93 Spotlight The Peacock Mantis Shrimp Odontodactylus scyllarus is - as its common name implies - the most colorful species among these widespread crustacean predators. THETHE LURKINGLURKING HORRORHORROR OFOF THETHE DEEPDEEP MANTISMANTIS SHRIMPSSHRIMPS Fast, flexible, fearless - scurrying and scooting among the coral rubble or suddenly exploding from their burrows in the muck. To impale and smash their hapless prey GOOGLE EARTH COORDINATES HERE 94 TEXT BY ANDREA FERRARI PHOTOS BY ANDREA & ANTONELLA FERRARI Wreathed any newcomers to scuba in a cloud of Mdiving are scared of sharks. Others are volcanic sand, a large afraid of morays. Some again are Lysiosquillina intimidated by barracudas... Little they sp. literally know that some of the scariest, most explodes from fearsome and probably most monstrous its burrow creatures of the deep lurk a few feet in a three- below the surface, silently waiting, millisecond coldly staring at their surroundings, attack. This is a “spearer” waiting for the opportunity to strike with species - notice a lightning-fast motion and to cruelly its sharply impale their prey or smash it to toothed smithereens! Luckily, most of these raptorial claws. terrifying critters are just a few inches long – otherwise diving on coral reefs might be a risky proposition indeed for every human being... But stop for a moment, and consider those cunning predators of the seabottom, the mantis shrimps: an elongated, segmented and armored body, capable of great flexibility and yet strong enough to resist the bite of all but the fiercest triggerfish; a series of short, parallel, jointed legs positioned under the thorax to swiftly propel it among the reefs rubble bottom; a pair of incredibly large, multifaceted dragonfly-like eyes, mounted on sophisticated swiveling joints, capable of giving the animal an absolutely unbeatable 3-D vision on a 360° field of vision, immensely better than our own and enabling it to strike with implacable accuracy at its chosen target. -

Stomatopoda (Crustacea: Hoplocarida) from the Shallow, Inshore Waters of the Northern Gulf of Mexico (Apalachicola River, Florida to Port Aransas, Texas)
Gulf and Caribbean Research Volume 16 Issue 1 January 2004 Stomatopoda (Crustacea: Hoplocarida) from the Shallow, Inshore Waters of the Northern Gulf of Mexico (Apalachicola River, Florida to Port Aransas, Texas) John M. Foster University of Southern Mississippi, [email protected] Brent P. Thoma University of Southern Mississippi Richard W. Heard University of Southern Mississippi, [email protected] Follow this and additional works at: https://aquila.usm.edu/gcr Part of the Marine Biology Commons Recommended Citation Foster, J. M., B. P. Thoma and R. W. Heard. 2004. Stomatopoda (Crustacea: Hoplocarida) from the Shallow, Inshore Waters of the Northern Gulf of Mexico (Apalachicola River, Florida to Port Aransas, Texas). Gulf and Caribbean Research 16 (1): 49-58. Retrieved from https://aquila.usm.edu/gcr/vol16/iss1/7 DOI: https://doi.org/10.18785/gcr.1601.07 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Gulf and Caribbean Research Vol 16, 49–58, 2004 Manuscript received December 15, 2003; accepted January 28, 2004 STOMATOPODA (CRUSTACEA: HOPLOCARIDA) FROM THE SHALLOW, INSHORE WATERS OF THE NORTHERN GULF OF MEXICO (APALACHICOLA RIVER, FLORIDA TO PORT ARANSAS, TEXAS) John M. Foster, Brent P. Thoma, and Richard W. Heard Department of Coastal Sciences, The University of Southern Mississippi, 703 East Beach Drive, Ocean Springs, Mississippi 39564, E-mail [email protected] (JMF), [email protected] (BPT), [email protected] (RWH) ABSTRACT Six species representing the order Stomatopoda are reported from the shallow, inshore waters (passes, bays, and estuaries) of the northern Gulf of Mexico limited to a depth of 10 m or less, and by the Apalachicola River (Florida) in the east and Port Aransas (Texas) in the west. -

Spineless Spineless Rachael Kemp and Jonathan E
Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Spineless Spineless Status and trends of the world’s invertebrates of the world’s Status and trends Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Disclaimer The designation of the geographic entities in this report, and the presentation of the material, do not imply the expressions of any opinion on the part of ZSL, IUCN or Wildscreen concerning the legal status of any country, territory, area, or its authorities, or concerning the delimitation of its frontiers or boundaries. Citation Collen B, Böhm M, Kemp R & Baillie JEM (2012) Spineless: status and trends of the world’s invertebrates. Zoological Society of London, United Kingdom ISBN 978-0-900881-68-8 Spineless: status and trends of the world’s invertebrates (paperback) 978-0-900881-70-1 Spineless: status and trends of the world’s invertebrates (online version) Editors Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Zoological Society of London Founded in 1826, the Zoological Society of London (ZSL) is an international scientifi c, conservation and educational charity: our key role is the conservation of animals and their habitats. www.zsl.org International Union for Conservation of Nature International Union for Conservation of Nature (IUCN) helps the world fi nd pragmatic solutions to our most pressing environment and development challenges. www.iucn.org Wildscreen Wildscreen is a UK-based charity, whose mission is to use the power of wildlife imagery to inspire the global community to discover, value and protect the natural world. -

Mantis Shrimp - Wikipedia
Mantis shrimp - Wikipedia https://en.wikipedia.org/wiki/Mantis_shrimp Mantis shrimp Mantis shrimps , or stomatopods , are marine crustaceans of the Mantis shrimp order Stomatopoda . Some species have specialised calcified "clubs" that can strike with great power, while others have sharp forelimbs used Temporal range: 400–0 Ma to capture prey. They branched from other members of the class Pre Є Є O S D C P T J K Pg N Malacostraca around 340 million years ago. [2] Mantis shrimps typically grow to around 10 cm (3.9 in) in length. A few can reach up to 38 cm (15 in). [3] The largest mantis shrimp ever caught had a length of 46 cm (18 in); it was caught in the Indian River near Fort Pierce, Florida, in the United States.[4] A mantis shrimp's carapace (the bony, thick shell that covers crustaceans and some other species) covers only the rear part of Odontodactylus scyllarus the head and the first four segments of the thorax. Varieties range from shades of brown to vivid colors, as more than 450 species of mantis Scientific classification shrimps are known. They are among the most important predators in Kingdom: Animalia many shallow, tropical and subtropical marine habitats. However, Phylum: Arthropoda despite being common, they are poorly understood, as many species spend most of their lives tucked away in burrows and holes. [5] Subphylum: Crustacea Called "sea locusts" by ancient Assyrians, "prawn killers" in Australia, [6] Class: Malacostraca and now sometimes referred to as "thumb splitters"—because of the Subclass: Hoplocarida [7] animal's ability to inflict painful gashes if handled incautiously Order: Stomatopoda —mantis shrimps have powerful claws that are used to attack and kill Latreille, 1817 prey by spearing, stunning, or dismembering. -

Power Amplification Strategies Across Animals
The University of Akron IdeaExchange@UAkron Williams Honors College, Honors Research The Dr. Gary B. and Pamela S. Williams Honors Projects College Spring 2021 Power Amplification Strategies Across Animals Rayhan Asif [email protected] Follow this and additional works at: https://ideaexchange.uakron.edu/honors_research_projects Part of the Biological and Chemical Physics Commons, Biomechanics Commons, Comparative and Evolutionary Physiology Commons, Entomology Commons, Motor Control Commons, and the Systems and Integrative Physiology Commons Please take a moment to share how this work helps you through this survey. Your feedback will be important as we plan further development of our repository. Recommended Citation Asif, Rayhan, "Power Amplification Strategies Across Animals" (2021). Williams Honors College, Honors Research Projects. 1340. https://ideaexchange.uakron.edu/honors_research_projects/1340 This Dissertation/Thesis is brought to you for free and open access by The Dr. Gary B. and Pamela S. Williams Honors College at IdeaExchange@UAkron, the institutional repository of The University of Akron in Akron, Ohio, USA. It has been accepted for inclusion in Williams Honors College, Honors Research Projects by an authorized administrator of IdeaExchange@UAkron. For more information, please contact [email protected], [email protected]. Evolution of Power Amplification Methods Rayhan Asif The University of Akron Abstract Animals use muscles for movement, but some have evolved mechanisms to exceed maximum power used in a motion known as power amplification. In this literature review, I analyzed and compared the evolution of structures capable of power amplification between species. Structures capable of power amplification were broken down into the basic components of the engine, amplifier, and tool. -

A New Fossil Mantis Shrimp and the Convergent Evolution of a Lobster-Like Morphotype
A new fossil mantis shrimp and the convergent evolution of a lobster-like morphotype Carolin Haug1,2 and Joachim T. Haug1,2 1 Biology II, Ludwig-Maximilians-Universität München, Planegg-Martinsried, Germany 2 GeoBio-Center, Ludwig-Maximilians-Universität München, Munich, Germany ABSTRACT Eumalacostracan crustaceans all have a more or less stereotypic body organisation in the sense of tagmosis. Originally, this included a head with six segments (ocular segment plus five appendage-bearing segments), a thorax region with eight segments, and a pleon with six segments. Interestingly, despite these restrictions in variability in terms of tagmosis, the morphological diversity within Eumalacostraca is rather high. A group providing representative examples that are commonly known is Decapoda. Decapodan crustaceans include shrimp-like forms, lobster-like forms and crab-like forms. The stem species of Eucarida, the group including Decapoda and Euphausiacea, presumably possessed a rather shrimp-like morphology, quite similar to the stem species of Eumalacostraca. Also two other lineages within Eumalacostraca, namely Hoplocarida (with the mantis shrimps as modern representatives) and Neocarida (with the sister groups Thermosbaenacea and Peracarida) evolved from the shrimp-like body organisation to include a lobster-like one. In this study, we demonstrate that the stepwise evolution towards a lobster morphotype occurred to a certain extent in similar order in these three lineages, Hoplocarida, Eucarida and Peracarida, leading to similar types of derived body organisation. This evolutionary reconstruction is based not only on observations of modern fauna, but especially on exceptionally preserved Mesozoic fossils, including the description of a new species of mantis shrimps bridging the morphological gap Submitted 21 August 2019 Accepted 26 February 2021 between the more ancestral-appearing Carboniferous forms and the more Published 16 April 2021 modern-appearing Jurassic forms. -

Ultraviolet Filters in Stomatopod Crustaceans: Diversity, Ecology and Evolution Michael J
© 2015. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2015) 218, 2055-2066 doi:10.1242/jeb.122036 RESEARCH ARTICLE Ultraviolet filters in stomatopod crustaceans: diversity, ecology and evolution Michael J. Bok*,§, Megan L. Porter‡ and Thomas W. Cronin ABSTRACT 1988; Marshall et al., 1991a) and at least five receptors sensitive to Stomatopod crustaceans employ unique ultraviolet (UV) optical filters various spectral ranges of ultraviolet (UV) light (Kleinlogel and in order to tune the spectral sensitivities of their UV-sensitive Marshall, 2009; Marshall and Oberwinkler, 1999). Underlying photoreceptors. In the stomatopod species Neogonodactylus oerstedii, these diverse visual sensitivities is an array of optical and retinal we previously found four filter types, produced by five distinct structural modifications (Horridge, 1978; Marshall et al., 1991a; mycosporine-like amino acid pigments in the crystalline cones of their Schiff et al., 1986), the expression of a great number of opsins specialized midband ommatidial facets. This UV-spectral tuning array resulting in the most visual pigments yet described in a single eye produces receptors with at least six distinct spectral sensitivities, (Cronin and Marshall, 1989b; Cronin et al., 1993; Porter et al., 2009, despite expressing only two visual pigments. Here, we present a 2013), and the tuning of spectral sensitivity via serial filtering broad survey of these UV filters across the stomatopod order, effects due to more distal visual pigments as well as photostable examining their spectral absorption properties in 21 species from colored pigments (Cronin and Marshall, 1989a; Cronin et al., seven families in four superfamilies. We found that UV filters are 1994a,b, 2014; Marshall, 1988; Marshall et al., 1991b; Porter et al., present in three of the four superfamilies, and evolutionary character 2010). -

Adult and Larval Stomatopod Crustaceans Occurring in Hawaiian Waters1
Adult and Larval Stomatopod Crustaceans Occurring in Hawaiian Waters1 SIDNEY JOSEPH TOWNSLEy2 INTRODUCTION t~on of the Bernice P. Bishop Museum, found Sl X genera and eight species from Hawaii: THE STOMATOPODA constitute the only order belonging to the division Hoplocarida of the Squilla oratoria, S. alba, Pseudosquilla ciliata, malacostracan Crustacea. All living forms are P. oculata, Coronida sinuosa, Lysiosquilla macu ~do~todactylus included in a single family, the Squillidae, lata,. hanseni, and Gonodactylus Since th at time no extensive studies whose characteristics are the same as those guerlnJ. of the order. The order, according to Bigelow have been made on specimens from Hawaii, (1894), may be defined as Crustacea which but occasional reports of previously recorded ~pecies fr~m have these characteristics : the stalked eyes and this region have found their way anrennules are borne upon distinct mo vable into .the ht.erature. In addition to these eight segments; the adult rostrum is separated from speCles which have been again collected dur the carapace by a movable joint; the carapace _ ing the progress of thi s study, I have found is small and does not cover the last four tho two species which have not been recorded fr~.m racic somites; the first five pairs of thoracic this region previousl y, namely, Squilla appendages are not biramous and serve as boops and an undescribed species of Squilla. accessory mouth parts, with the second pair Stomatopods are usually found in very shal strongly developed into large raptorial limbs ; low water, but some species occur at greater the last three pairs of thoracic appendages depths. -
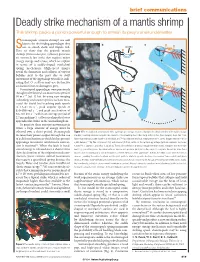
Deadly Strike Mechanism of a Mantis Shrimp This Shrimp Packs a Punch Powerful Enough to Smash Its Prey’S Shell Underwater
22.4 Brief comms MH 15/4/04 5:40 pm Page 819 brief communications Deadly strike mechanism of a mantis shrimp This shrimp packs a punch powerful enough to smash its prey’s shell underwater. tomatopods (mantis shrimp) are well known for the feeding appendages they a b c Suse to smash shells and impale fish. Saddle Here we show that the peacock mantis shrimp (Odontodactylus scyllarus) generates c an extremely fast strike that requires major v energy storage and release, which we explain 0 ms m in terms of a saddle-shaped exoskeletal spring mechanism. High-speed images p reveal the formation and collapse of vapour bubbles next to the prey due to swift d movement of the appendage towards it, indi- 1 ms cating that O. scyllarus may use destructive cavitation forces to damage its prey. Stomatopod appendages were previously thought to be limited to a maximum speed of 10msǁ1 (ref. 1) but, by using new imaging technology and a faster species,we have mea- 2 ms sured the dactyl heel reaching peak speeds of 14–23 m sǁ1,peak angular speeds of 670–990 rad sǁ1, and peak acceleration of 65–104 km sǁ2 within an average period of 2.7ms,making O.scyllarusperhaps the fastest 3 ms appendicular striker in the animal kingdom. To generate these extreme movements in water, a large amount of energy must be released over a short period. Stomatopods Figure 1 The mechanics of a stomatopod strike. a, A high-speed image sequence illustrates the distal extension of the saddle (orange increase their power output through the use triangles) occurring simultaneously with the extension of the smashing heel of the dactyl and propodus (blue triangles).