Linkage Mechanics and Power Amplification of the Mantis Shrimp's
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Learning in Stomatopod Crustaceans
International Journal of Comparative Psychology, 2006, 19 , 297-317. Copyright 2006 by the International Society for Comparative Psychology Learning in Stomatopod Crustaceans Thomas W. Cronin University of Maryland Baltimore County, U.S.A. Roy L. Caldwell University of California, Berkeley, U.S.A. Justin Marshall University of Queensland, Australia The stomatopod crustaceans, or mantis shrimps, are marine predators that stalk or ambush prey and that have complex intraspecific communication behavior. Their active lifestyles, means of predation, and intricate displays all require unusual flexibility in interacting with the world around them, imply- ing a well-developed ability to learn. Stomatopods have highly evolved sensory systems, including some of the most specialized visual systems known for any animal group. Some species have been demonstrated to learn how to recognize and use novel, artificial burrows, while others are known to learn how to identify novel prey species and handle them for effective predation. Stomatopods learn the identities of individual competitors and mates, using both chemical and visual cues. Furthermore, stomatopods can be trained for psychophysical examination of their sensory abilities, including dem- onstration of color and polarization vision. These flexible and intelligent invertebrates continue to be attractive subjects for basic research on learning in animals with relatively simple nervous systems. Among the most captivating of all arthropods are the stomatopod crusta- ceans, or mantis shrimps. These marine creatures, unfamiliar to most biologists, are abundant members of shallow marine ecosystems, where they are often the dominant invertebrate predators. Their common name refers to their method of capturing prey using a folded, anterior raptorial appendage that looks superficially like the foreleg of a praying mantis. -

MANTIS SHRIMPSSHRIMPS Fast, Flexible, Fearless - Scurrying and Scooting Among the Coral Rubble Or Suddenly Exploding from Their Burrows in the Muck
93 Spotlight The Peacock Mantis Shrimp Odontodactylus scyllarus is - as its common name implies - the most colorful species among these widespread crustacean predators. THETHE LURKINGLURKING HORRORHORROR OFOF THETHE DEEPDEEP MANTISMANTIS SHRIMPSSHRIMPS Fast, flexible, fearless - scurrying and scooting among the coral rubble or suddenly exploding from their burrows in the muck. To impale and smash their hapless prey GOOGLE EARTH COORDINATES HERE 94 TEXT BY ANDREA FERRARI PHOTOS BY ANDREA & ANTONELLA FERRARI Wreathed any newcomers to scuba in a cloud of Mdiving are scared of sharks. Others are volcanic sand, a large afraid of morays. Some again are Lysiosquillina intimidated by barracudas... Little they sp. literally know that some of the scariest, most explodes from fearsome and probably most monstrous its burrow creatures of the deep lurk a few feet in a three- below the surface, silently waiting, millisecond coldly staring at their surroundings, attack. This is a “spearer” waiting for the opportunity to strike with species - notice a lightning-fast motion and to cruelly its sharply impale their prey or smash it to toothed smithereens! Luckily, most of these raptorial claws. terrifying critters are just a few inches long – otherwise diving on coral reefs might be a risky proposition indeed for every human being... But stop for a moment, and consider those cunning predators of the seabottom, the mantis shrimps: an elongated, segmented and armored body, capable of great flexibility and yet strong enough to resist the bite of all but the fiercest triggerfish; a series of short, parallel, jointed legs positioned under the thorax to swiftly propel it among the reefs rubble bottom; a pair of incredibly large, multifaceted dragonfly-like eyes, mounted on sophisticated swiveling joints, capable of giving the animal an absolutely unbeatable 3-D vision on a 360° field of vision, immensely better than our own and enabling it to strike with implacable accuracy at its chosen target. -

Spineless Spineless Rachael Kemp and Jonathan E
Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Spineless Spineless Status and trends of the world’s invertebrates of the world’s Status and trends Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Disclaimer The designation of the geographic entities in this report, and the presentation of the material, do not imply the expressions of any opinion on the part of ZSL, IUCN or Wildscreen concerning the legal status of any country, territory, area, or its authorities, or concerning the delimitation of its frontiers or boundaries. Citation Collen B, Böhm M, Kemp R & Baillie JEM (2012) Spineless: status and trends of the world’s invertebrates. Zoological Society of London, United Kingdom ISBN 978-0-900881-68-8 Spineless: status and trends of the world’s invertebrates (paperback) 978-0-900881-70-1 Spineless: status and trends of the world’s invertebrates (online version) Editors Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Zoological Society of London Founded in 1826, the Zoological Society of London (ZSL) is an international scientifi c, conservation and educational charity: our key role is the conservation of animals and their habitats. www.zsl.org International Union for Conservation of Nature International Union for Conservation of Nature (IUCN) helps the world fi nd pragmatic solutions to our most pressing environment and development challenges. www.iucn.org Wildscreen Wildscreen is a UK-based charity, whose mission is to use the power of wildlife imagery to inspire the global community to discover, value and protect the natural world. -

Molecular Diversity of Visual Pigments in Stomatopoda (Crustacea)
Visual Neuroscience (2009), 26, 255–265. Printed in the USA. Copyright Ó 2009 Cambridge University Press 0952-5238/09 $25.00 doi:10.1017/S0952523809090129 Molecular diversity of visual pigments in Stomatopoda (Crustacea) MEGAN L. PORTER, MICHAEL J. BOK, PHYLLIS R. ROBINSON AND THOMAS W. CRONIN Department of Biological Sciences, University of Maryland, Baltimore County, Baltimore, Maryland (RECEIVED February 16, 2009; ACCEPTED May 11, 2009; FIRST PUBLISHED ONLINE June 18, 2009) Abstract Stomatopod crustaceans possess apposition compound eyes that contain more photoreceptor types than any other animal described. While the anatomy and physiology of this complexity have been studied for more than two decades, few studies have investigated the molecular aspects underlying the stomatopod visual complexity. Based on previous studies of the structure and function of the different types of photoreceptors, stomatopod retinas are hypothesized to contain up to 16 different visual pigments, with 6 of these having sensitivity to middle or long wavelengths of light. We investigated stomatopod middle- and long-wavelength-sensitive opsin genes from five species with the hypothesis that each species investigated would express up to six different opsin genes. In order to understand the evolution of this class of stomatopod opsins, we examined the complement of expressed transcripts in the retinas of species representing a broad taxonomic range (four families and three superfamilies). A total of 54 unique retinal opsins were isolated, resulting in 6–15 different expressed transcripts in each species. Phylogenetically, these transcripts form six distinct clades, grouping with other crustacean opsins and sister to insect long-wavelength visual pigments. Within these stomatopod opsin groups, intra- and interspecific clusters of highly similar transcripts suggest that there has been rampant recent gene duplication. -

Folk Taxonomy of Marine Fauna on Takuu Atoll, Papua New Guinea
2 SPC Traditional Marine Resource Management and Knowledge Information Bulletin #39 – April 2018 Catching names: Folk taxonomy of marine fauna on Takuu Atoll, Papua New Guinea Anke Moesinger1 Abstract Folk taxonomies are a critical component for understanding resource use patterns and cultural, social and economic preferences on geographically remote Pacific atolls. To understand how people perceive and make use of their environment, 200 local names for marine vertebrates and invertebrates were collected and the hierarchical classification system was documented on Takuu Atoll in Papua New Guinea. The local nomenclature of the marine fauna of Takuu is based largely on shared fundamental morphological charac- teristics. Furthermore, all fish (Te ika) in the ocean are placed into one of five distinct groups in the hierar- chical classification system. These include three functional groups that are categorised by ecological niche, whereas another group encompasses all fish that possess a certain behavioural trait. The fifth group is unique in that it is solely made up of fish that were previously targeted during local Sii fishing expeditions. This article presents an analysis of Takuu residents’ descriptions and classifications of local fish and marine invertebrates. Keywords Folk taxonomy, Takuu Atoll, local knowledge, Polynesian outlier, folk hierarchical classification Introduction atoll is one of only three Polynesian outliers found in PNG. The others include Nukuria, also known as Takuu Atoll islanders are dependent on and inex- Fead Island, which is located 160 km to the north- tricably linked to the marine environment that sur- west of the atoll, and Nukumanu, or Tasman, which rounds them, and fishing permeates almost every is situated 315 km to the east. -

Polarization Signals in Mantis Shrimps
Polarization signals in mantis shrimps Thomas W. Cronin*a, Tsyr-Huei Chioua,b, Roy L. Caldwellc, Nicholas Robertsd, Justin Marshallb aDept. of Biological Sciences, UMBC, Baltimore, MD, USA 21250; bQBI, Univ. of Queensland, Brisbane, Australia 4072; cDept. of Integrative Biology, Univ. of California, Berkeley, CA 94720; dThe Photon Science Institute, Univ. of Manchester, Manchester, UK M13 9PL ABSTRACT While color signals are well known as a form of animal communication, a number of animals communicate using signals based on patterns of polarized light reflected from specialized body parts or structures. Mantis shrimps, a group of marine crustaceans, have evolved a great diversity of such signals, several of which are based on photonic structures. These include resonant scattering devices, structures based on layered dichroic molecules, and structures that use birefringent layers to produce circular polarization. Such biological polarizers operate in different spectral regions ranging from the near-UV to medium wavelengths of visible light. In addition to the structures that are specialized for signal production, the eyes of many species of mantis shrimp are adapted to detect linearly polarized light in the ultraviolet and in the green, using specialized sets of photoreceptors with oriented, dichroic visual pigments. Finally, a few mantis shrimp species produce biophotonic retarders within their photoreceptors that permit the detection of circularly polarized light and are thus the only animals known to sense this form of polarization. Mantis shrimps use polarized light in species-specific signals related to mating and territorial defense, and their means of manipulating light’s polarization can inspire designs for artificial polarizers and achromatic retarders. -

A New Fossil Mantis Shrimp and the Convergent Evolution of a Lobster-Like Morphotype
A new fossil mantis shrimp and the convergent evolution of a lobster-like morphotype Carolin Haug1,2 and Joachim T. Haug1,2 1 Biology II, Ludwig-Maximilians-Universität München, Planegg-Martinsried, Germany 2 GeoBio-Center, Ludwig-Maximilians-Universität München, Munich, Germany ABSTRACT Eumalacostracan crustaceans all have a more or less stereotypic body organisation in the sense of tagmosis. Originally, this included a head with six segments (ocular segment plus five appendage-bearing segments), a thorax region with eight segments, and a pleon with six segments. Interestingly, despite these restrictions in variability in terms of tagmosis, the morphological diversity within Eumalacostraca is rather high. A group providing representative examples that are commonly known is Decapoda. Decapodan crustaceans include shrimp-like forms, lobster-like forms and crab-like forms. The stem species of Eucarida, the group including Decapoda and Euphausiacea, presumably possessed a rather shrimp-like morphology, quite similar to the stem species of Eumalacostraca. Also two other lineages within Eumalacostraca, namely Hoplocarida (with the mantis shrimps as modern representatives) and Neocarida (with the sister groups Thermosbaenacea and Peracarida) evolved from the shrimp-like body organisation to include a lobster-like one. In this study, we demonstrate that the stepwise evolution towards a lobster morphotype occurred to a certain extent in similar order in these three lineages, Hoplocarida, Eucarida and Peracarida, leading to similar types of derived body organisation. This evolutionary reconstruction is based not only on observations of modern fauna, but especially on exceptionally preserved Mesozoic fossils, including the description of a new species of mantis shrimps bridging the morphological gap Submitted 21 August 2019 Accepted 26 February 2021 between the more ancestral-appearing Carboniferous forms and the more Published 16 April 2021 modern-appearing Jurassic forms. -

Ultraviolet Filters in Stomatopod Crustaceans: Diversity, Ecology and Evolution Michael J
© 2015. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2015) 218, 2055-2066 doi:10.1242/jeb.122036 RESEARCH ARTICLE Ultraviolet filters in stomatopod crustaceans: diversity, ecology and evolution Michael J. Bok*,§, Megan L. Porter‡ and Thomas W. Cronin ABSTRACT 1988; Marshall et al., 1991a) and at least five receptors sensitive to Stomatopod crustaceans employ unique ultraviolet (UV) optical filters various spectral ranges of ultraviolet (UV) light (Kleinlogel and in order to tune the spectral sensitivities of their UV-sensitive Marshall, 2009; Marshall and Oberwinkler, 1999). Underlying photoreceptors. In the stomatopod species Neogonodactylus oerstedii, these diverse visual sensitivities is an array of optical and retinal we previously found four filter types, produced by five distinct structural modifications (Horridge, 1978; Marshall et al., 1991a; mycosporine-like amino acid pigments in the crystalline cones of their Schiff et al., 1986), the expression of a great number of opsins specialized midband ommatidial facets. This UV-spectral tuning array resulting in the most visual pigments yet described in a single eye produces receptors with at least six distinct spectral sensitivities, (Cronin and Marshall, 1989b; Cronin et al., 1993; Porter et al., 2009, despite expressing only two visual pigments. Here, we present a 2013), and the tuning of spectral sensitivity via serial filtering broad survey of these UV filters across the stomatopod order, effects due to more distal visual pigments as well as photostable examining their spectral absorption properties in 21 species from colored pigments (Cronin and Marshall, 1989a; Cronin et al., seven families in four superfamilies. We found that UV filters are 1994a,b, 2014; Marshall, 1988; Marshall et al., 1991b; Porter et al., present in three of the four superfamilies, and evolutionary character 2010). -

Adult and Larval Stomatopod Crustaceans Occurring in Hawaiian Waters1
Adult and Larval Stomatopod Crustaceans Occurring in Hawaiian Waters1 SIDNEY JOSEPH TOWNSLEy2 INTRODUCTION t~on of the Bernice P. Bishop Museum, found Sl X genera and eight species from Hawaii: THE STOMATOPODA constitute the only order belonging to the division Hoplocarida of the Squilla oratoria, S. alba, Pseudosquilla ciliata, malacostracan Crustacea. All living forms are P. oculata, Coronida sinuosa, Lysiosquilla macu ~do~todactylus included in a single family, the Squillidae, lata,. hanseni, and Gonodactylus Since th at time no extensive studies whose characteristics are the same as those guerlnJ. of the order. The order, according to Bigelow have been made on specimens from Hawaii, (1894), may be defined as Crustacea which but occasional reports of previously recorded ~pecies fr~m have these characteristics : the stalked eyes and this region have found their way anrennules are borne upon distinct mo vable into .the ht.erature. In addition to these eight segments; the adult rostrum is separated from speCles which have been again collected dur the carapace by a movable joint; the carapace _ ing the progress of thi s study, I have found is small and does not cover the last four tho two species which have not been recorded fr~.m racic somites; the first five pairs of thoracic this region previousl y, namely, Squilla appendages are not biramous and serve as boops and an undescribed species of Squilla. accessory mouth parts, with the second pair Stomatopods are usually found in very shal strongly developed into large raptorial limbs ; low water, but some species occur at greater the last three pairs of thoracic appendages depths. -

Clam Ctenoides Ales (Finlay, 1927): Mechanisms and Behavioral Function
Flashing in the 'Disco' Clam Ctenoides ales (Finlay, 1927): Mechanisms and Behavioral Function By Lindsey Dougherty Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Roy L. Caldwell, Chair Professor David R. Lindberg Professor Damian O. Elias Spring 2016 Abstract Flashing in the 'Disco' Clam Ctenoides ales (Finlay, 1927): Mechanisms and Behavioral Function by Lindsey Dougherty Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Roy L. Caldwell, Chair This dissertation investigated the ‘disco’ clam Ctenoides ales (Limidae), which is the only bivalve in the world that has a behaviorally-mediated flashing display. Topics covered include (i) mechanisms, ultrastructure and movement that produce the flashing, (ii) the fitness value (function) of the flashing, (iii) the clams’ sensory abilities and vision, and (iv) the clams’ ecology, distribution and habitat. The flashing occurs on the clams’ mantle lip. Electron microscopy revealed two distinct tissue sides; one highly scattering side that contains dense aggregations of spheres composed of silica (white), and one highly absorbing side that does not (red). High-speed video confirmed that the two sides alternate rapidly, creating the appearance of flashing. Optical modeling suggested that the sphere’s diameter is nearly optimal for scattering light, especially at shorter wavelengths, which predominate in the ocean. This simple mechanism produces a striking optical effect. Three potential hypotheses for the fitness value of the flashing were investigated; conspecific attraction, prey luring, and/or predator deterrence. The lack of movement toward other C. -

Exceptional Diversity of Opsin Expression Patterns in Neogonodactylus Oerstedii (Stomatopoda) Retinas
Exceptional diversity of opsin expression patterns in Neogonodactylus oerstedii (Stomatopoda) retinas Megan L. Portera,b,1,2, Hiroko Awataa,1, Michael J. Boka,c, and Thomas W. Cronina aDepartment of Biological Sciences, University of Maryland Baltimore County, Baltimore, MD 21250; bDepartment of Biology, University of Hawai’iat Manoa, Honolulu, HI 96822; and cSchool of Biological Sciences, University of Bristol, BS8 1TQ Bristol, United Kingdom Edited by Claude Desplan, New York University, New York, NY, and approved February 27, 2020 (received for review October 4, 2019) Stomatopod crustaceans possess some of the most complex animal Most of the spectral diversity found in stomatopod retinas visual systems, including at least 16 spectrally distinct types of exists in the four dorsal-most ommatidial MB rows (MB rows 1 photoreceptive units (e.g., assemblages of photoreceptor cells). Here to 4). In MB rows 1 to 4, the seven proximal photoreceptor cells we fully characterize the set of opsin genes expressed in retinal tissues (R1–7) are subdivided into two separate photoreceptive units, and determine expression patterns of each in the stomatopod Neo- formed by sets of three or four retinula cells. Therefore, in MB gonodactylus oerstedii. Using a combination of transcriptome and rows 1 to 4 each ommatidium is formed from three successive RACE sequencing, we identified 33 opsin transcripts expressed in each photoreceptive layers comprising the distal UVS R8 photore- N. oerstedii eye, which are predicted to form 20 long-wavelength– ceptor, and middle and proximal photoreceptive layers sensitive sensitive, 10 middle-wavelength–sensitive, and three UV-sensitive to the visible spectrum (Fig. -
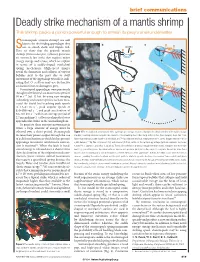
Deadly Strike Mechanism of a Mantis Shrimp This Shrimp Packs a Punch Powerful Enough to Smash Its Prey’S Shell Underwater
22.4 Brief comms MH 15/4/04 5:40 pm Page 819 brief communications Deadly strike mechanism of a mantis shrimp This shrimp packs a punch powerful enough to smash its prey’s shell underwater. tomatopods (mantis shrimp) are well known for the feeding appendages they a b c Suse to smash shells and impale fish. Saddle Here we show that the peacock mantis shrimp (Odontodactylus scyllarus) generates c an extremely fast strike that requires major v energy storage and release, which we explain 0 ms m in terms of a saddle-shaped exoskeletal spring mechanism. High-speed images p reveal the formation and collapse of vapour bubbles next to the prey due to swift d movement of the appendage towards it, indi- 1 ms cating that O. scyllarus may use destructive cavitation forces to damage its prey. Stomatopod appendages were previously thought to be limited to a maximum speed of 10msǁ1 (ref. 1) but, by using new imaging technology and a faster species,we have mea- 2 ms sured the dactyl heel reaching peak speeds of 14–23 m sǁ1,peak angular speeds of 670–990 rad sǁ1, and peak acceleration of 65–104 km sǁ2 within an average period of 2.7ms,making O.scyllarusperhaps the fastest 3 ms appendicular striker in the animal kingdom. To generate these extreme movements in water, a large amount of energy must be released over a short period. Stomatopods Figure 1 The mechanics of a stomatopod strike. a, A high-speed image sequence illustrates the distal extension of the saddle (orange increase their power output through the use triangles) occurring simultaneously with the extension of the smashing heel of the dactyl and propodus (blue triangles).