RESEARCH ARTICLE Comparative Spring Mechanics in Mantis Shrimp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Learning in Stomatopod Crustaceans
International Journal of Comparative Psychology, 2006, 19 , 297-317. Copyright 2006 by the International Society for Comparative Psychology Learning in Stomatopod Crustaceans Thomas W. Cronin University of Maryland Baltimore County, U.S.A. Roy L. Caldwell University of California, Berkeley, U.S.A. Justin Marshall University of Queensland, Australia The stomatopod crustaceans, or mantis shrimps, are marine predators that stalk or ambush prey and that have complex intraspecific communication behavior. Their active lifestyles, means of predation, and intricate displays all require unusual flexibility in interacting with the world around them, imply- ing a well-developed ability to learn. Stomatopods have highly evolved sensory systems, including some of the most specialized visual systems known for any animal group. Some species have been demonstrated to learn how to recognize and use novel, artificial burrows, while others are known to learn how to identify novel prey species and handle them for effective predation. Stomatopods learn the identities of individual competitors and mates, using both chemical and visual cues. Furthermore, stomatopods can be trained for psychophysical examination of their sensory abilities, including dem- onstration of color and polarization vision. These flexible and intelligent invertebrates continue to be attractive subjects for basic research on learning in animals with relatively simple nervous systems. Among the most captivating of all arthropods are the stomatopod crusta- ceans, or mantis shrimps. These marine creatures, unfamiliar to most biologists, are abundant members of shallow marine ecosystems, where they are often the dominant invertebrate predators. Their common name refers to their method of capturing prey using a folded, anterior raptorial appendage that looks superficially like the foreleg of a praying mantis. -

The Role of Neaxius Acanthus
Wattenmeerstation Sylt The role of Neaxius acanthus (Thalassinidea: Strahlaxiidae) and its burrows in a tropical seagrass meadow, with some remarks on Corallianassa coutierei (Thalassinidea: Callianassidae) Diplomarbeit Institut für Biologie / Zoologie Fachbereich Biologie, Chemie und Pharmazie Freie Universität Berlin vorgelegt von Dominik Kneer Angefertigt an der Wattenmeerstation Sylt des Alfred-Wegener-Instituts für Polar- und Meeresforschung in der Helmholtz-Gemeinschaft In Zusammenarbeit mit dem Center for Coral Reef Research der Hasanuddin University Makassar, Indonesien Sylt, Mai 2006 1. Gutachter: Prof. Dr. Thomas Bartolomaeus Institut für Biologie / Zoologie Freie Universität Berlin Berlin 2. Gutachter: Prof. Dr. Walter Traunspurger Fakultät für Biologie / Tierökologie Universität Bielefeld Bielefeld Meinen Eltern (wem sonst…) Table of contents 4 Abstract ...................................................................................................................................... 6 Zusammenfassung...................................................................................................................... 8 Abstrak ..................................................................................................................................... 10 Abbreviations ........................................................................................................................... 12 1 Introduction .......................................................................................................................... -
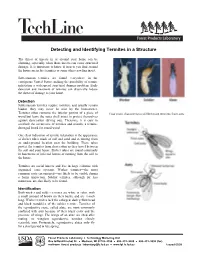
Termites in a Structure
Detecting and Identifying Termites in a Structure The threat of insects in or around your home can be alarming, especially when those insects can cause structural damage. It is important to know if insects you find around the house are in fact termites or some other crawling insect. Subterranean termites are found everywhere in the contiguous United States, making the possibility of termite infestation a widespread structural damage problem. Early detection and treatment of termites can drastically reduce the threat of damage to your home. Detection Subterranean termites require moisture and usually remain hidden—they may never be seen by the homeowner. Termites often consume the interior portion of a piece of Four main characteristics differentiate termites from ants: wood but leave the outer shell intact to protect themselves against desiccation (drying out). Therefore, it is easy to overlook the occurrence of termites and mistake a termite- damaged board for sound wood. One clear indication of termite infestation is the appearance of shelter tubes made of soil and sand and stemming from an underground location near the building. These tubes protect the termites from desiccation as they travel between the soil and your house. Shelter tubes are found commonly in basements of infected homes or running from the soil to the house. Termites are social insects and live in large colonies with organized caste systems. Worker termites—the most common caste encountered—are likely to be visible during a home inspection. Soldier termites, although far less numerous, are also likely to be found. Identification Both worker and soldier termites are white in color, with a small amount of brown on their backs, and are ¼-inch long. -

Stomatopod Interrelationships: Preliminary Results Based on Analysis of Three Molecular Loci
Arthropod Systematics & Phylogeny 91 67 (1) 91 – 98 © Museum für Tierkunde Dresden, eISSN 1864-8312, 17.6.2009 Stomatopod Interrelationships: Preliminary Results Based on Analysis of three Molecular Loci SHANE T. AHYONG 1 & SIMON N. JARMAN 2 1 Marine Biodiversity and Biodescurity, National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington, New Zealand [[email protected]] 2 Australian Antarctic Division, 203 Channel Highway, Kingston, Tasmania 7050, Australia [[email protected]] Received 16.iii.2009, accepted 15.iv.2009. Published online at www.arthropod-systematics.de on 17.vi.2009. > Abstract The mantis shrimps (Stomatopoda) are quintessential marine predators. The combination of powerful raptorial appendages and remarkably developed sensory systems place the stomatopods among the most effi cient invertebrate predators. High level phylogenetic analyses have been so far based on morphology. Crown-group Unipeltata appear to have diverged in two broad directions from the outset – one towards highly effi cient ‘spearing’ with multispinous dactyli on the raptorial claws (dominated by Lysiosquilloidea and Squilloidea), and the other towards ‘smashing’ (Gonodactyloidea). In a preliminary molecular study of stomatopod interrelationships, we assemble molecular data for mitochondrial 12S and 16S regions, combined with new sequences from the 16S and two regions of the nuclear 28S rDNA to compare with morphological hypotheses. Nineteen species representing 9 of 17 extant families and 3 of 7 superfamilies were analysed. The molecular data refl ect the overall patterns derived from morphology, especially in a monophyletic Squilloidea, a monophyletic Lysiosquilloidea and a monophyletic clade of gonodactyloid smashers. Molecular analyses, however, suggest the novel possibility that Hemisquillidae and possibly Pseudosquillidae, rather than being basal or near basal in Gonodactyloidea, may be basal overall to the extant stomatopods. -

03 Literature Review
- Literature Review: - Sr. No .1 - Name of the Author : P.G. Koehler and C. L. Tucker - Name of the Article : Subterranean Termite - Pages: 7 Nos. - Date of Published : April 1993 Revised in 2003 University of Florida, IFAS, Extention No. ENY 210 - Journal Name : Entomology and Nematology, Florida Cooperative Service - Review: Subterranean Termite, Dampwood and Drywood Termite are the main three types of termites . Subterranean Termites are the most destructive and it attacks structures by building tubes from nest to wood structures. They feed on wood which contain cellulose which is digested by them by protozoa and convert cellulose into usable food. The nest in the soil contains moisture to protect them from low humidity and predation and they build mud tunnels or tubes to reach wood. At swarming stage the broken wings are located at light are hence they are attracted to light. No wood contact with soil, ventilation, regular inspection can avoid the entry of termite and for effective control for Subterranean termite, Pre and post treatment methods for new and for existing structures at different stages with repellent and non repellent insecticides as required with the additional control method of using Termite Bait. - Sr. No . 2 - Name of the Author : Matthew R. Tarver, Christopher B. Florane, Dunhua Zhang, Casey Grimm and Alan R. Lax - Name of the Article : Methoprene and temperature effects on caste differentiation and protein composition in the Formosan Subterranean termite, Coptotermesfomosanus - Pages: 10 Nos. - Date of Published : December 2011 - Journal Name : Journal of Insect Science, Vol. 12/ article 18 - Review: Worker to soldier differentiation is modulated by temperature i.e. -
Colour Transcript
Colour Transcript Date: Wednesday, 30 March 2011 - 6:00PM Location: Museum of London 30 March 2011 Colour Professor William Ayliffe Some of you in this audience will be aware that it is the 150th anniversary of the first colour photograph, which was projected at a lecture at the Royal Institute by James Clerk Maxwell. This is the photograph, showing a tartan ribbon, which was taken using the first SLR, invented by Maxwell’s friend. He took three pictures, using three different filters, and was then able to project this gorgeous image, showing three different colours for the first time ever. Colour and Colour Vision This lecture is concerned with the questions: “What is colour?” and “What is colour vision?” - not necessarily the same things. We are going to look at train crashes and colour blindness (which is quite gruesome); the antique use of colour in pigments – ancient red Welsh “Ladies”; the meaning of colour in medieval Europe; discovery of new pigments; talking about colour; language and colour; colour systems and the psychology of colour. So there is a fair amount of ground to cover here, which is appropriate because colour is probably one of the most complex issues that we deal with. The main purpose of this lecture is to give an overview of the whole field of colour, without going into depth with any aspects in particular. Obviously, colour is a function of light because, without light, we cannot see colour. Light is that part of the electromagnetic spectrum that we can see, and that forms only a tiny portion. -

JCPA-S-19-00140.Pdf
This is a post-peer-review, pre-copyedit version of an article published in Journal of Comparative Physiology A. The final authenticated version is available online at: https://doi.org/10.1007/s00359-019-01387-5. Access to this work was provided by the University of Maryland, Baltimore County (UMBC) ScholarWorks@UMBC digital repository on the Maryland Shared Open Access (MD-SOAR) platform. Please provide feedback Please support the ScholarWorks@UMBC repository by emailing [email protected] and telling us what having access to this work means to you and why it’s important to you. Thank you. Journal of Comparative Physiology A Optic lobe organization in stomatopod crustacean species having different degrees of retinal complexity --Manuscript Draft-- Manuscript Number: Full Title: Optic lobe organization in stomatopod crustacean species having different degrees of retinal complexity Article Type: S.I. : Visual Circuits Funding Information: Air Force Office of Scientific Research Dr. Thomas W Cronin (FA9550-18-1-0278) Abstract: Stomatopod crustaceans possess tripartite compound eyes; upper and lower hemispheres are separated by an equatorial midband of several ommatidial rows. The organization of stomatopod retinas is well studied, but their optic lobes are less understood. We used histological staining, immunolabeling, and fluorescent tracer injections to compare optic lobes in two 6-midband-row species, Neogonodactylus oerstedii and Pseudosquilla ciliata , to those in two 2-midband-row species, Squilla empusa and Alima pacifica . Compared to the 6-row species, we found structural simplification in all optic neuropils in both 2-row species. Photoreceptor axons from 2- row midband ommatidia supply two sets of enlarged lamina cartridges, but a gap in the lamina exists at the location where the cartridges of the dorsal four ommatidial rows of 6-row species would appear. -

Wetlands Invertebrates Banded Woollybear(Isabella Tiger Moth Larva)
Wetlands Invertebrates Banded Woollybear (Isabella Tiger Moth larva) basics The banded woollybear gets its name for two reasons: its furry appearance and the fact that, like a bear, it hibernates during the winter. Woollybears are the caterpillar stage of medium sized moths known as tiger moths. This family of moths rivals butterflies in beauty and grace. There are approximately 260 species of tiger moths in North America. Though the best-known woollybear is the banded woollybear, there are at least 8 woollybear species in the U.S. with similar dense, bristly hair covering their bodies. Woollybears are most commonly seen in the autumn, when they are just about finished with feeding for the year. It is at this time that they seek out a place to spend the winter in hibernation. They have been eating various green plants since June or early July to gather enough energy for their eventual transformation into butterflies. A full-grown banded woollybear caterpillar is nearly two inches long and covered with tubercles from which arise stiff hairs of about equal length. Its body has 13 segments. Middle segments are covered with red-orange hairs and the anterior and posterior ends with black hairs. The orange-colored oblongs visible between the tufts of setae (bristly hairs) are spiracles—entrances to the respiratory system. Hair color and band width are highly variable; often as the caterpillar matures, black hairs (especially at the posterior end) are replaced with orange hairs. In general, older caterpillars have more black than young ones. However, caterpillars that fed and grew in an area where the fall weather was wetter tend to have more black hair than caterpillars from dry areas. -

Lesson 3 Life Cycles of Insects
Praying Mantis 3A-1 Hi, boys and girls. It’s time to meet one of the most fascinating insects on the planet. That’s me. I’m a praying mantis, named for the way I hold my two front legs together as though I am praying. I might look like I am praying, but my incredibly fast front legs are designed to grab my food in the blink of an eye! Praying Mantis 3A-1 I’m here to talk to you about the life stages of insects—how insects develop from birth to adult. Many insects undergo a complete change in shape and appearance. I’m sure that you are already familiar with how a caterpillar changes into a butterfly. The name of the process in which a caterpillar changes, or morphs, into a butterfly is called metamorphosis. Life Cycle of a Butterfly 3A-2 Insects like the butterfly pass through four stages in their life cycles: egg, larva [LAR-vah], pupa, and adult. Each stage looks completely different from the next. The young never resemble, or look like, their parents and almost always eat something entirely different. Life Cycle of a Butterfly 3A-2 The female insect lays her eggs on a host plant. When the eggs hatch, the larvae [LAR-vee] that emerge look like worms. Different names are given to different insects in this worm- like stage, and for the butterfly, the larva state is called a caterpillar. Insect larvae: maggot, grub and caterpillar3A-3 Fly larvae are called maggots; beetle larvae are called grubs; and the larvae of butterflies and moths, as you just heard, are called caterpillars. -

Research in Biological Control of the Formosan Subterranean Termite Cai Wang Louisiana State University and Agricultural and Mechanical College, [email protected]
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2014 Research in Biological Control of the Formosan Subterranean Termite Cai Wang Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Entomology Commons Recommended Citation Wang, Cai, "Research in Biological Control of the Formosan Subterranean Termite" (2014). LSU Doctoral Dissertations. 598. https://digitalcommons.lsu.edu/gradschool_dissertations/598 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. RESEARCH IN BIOLOGICAL CONTROL OF THE FORMOSAN SUBTERRANEAN TERMITE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Cai Wang M.S., Chinese Academy of Science, 2010 B.S., Huazhong University of Science and Technology, 2007 August 2014 ACKNOWLEDGMENTS I would like to express my sincerest appreciation to my major professor, Dr. Gregg Henderson, a very important person in my life. I am very impressive for his meticulous attitude for scientific research. I benefited greatly from his valuable and illuminating suggestions for my research. It is also very touching for Dr. Henderson’s patience for my preliminary and sometimes “crazy” ideas. Also, he always could “see” what I ignored. For example, when I unintentionally talked about an observation that soldier and worker termites run in different directions after disturbance, he immediately pointed out the potential value to continue studying this and gave me valuable suggestions in the experiment. -

Stomatopoda (Crustacea: Hoplocarida) from the Shallow, Inshore Waters of the Northern Gulf of Mexico (Apalachicola River, Florida to Port Aransas, Texas)
Gulf and Caribbean Research Volume 16 Issue 1 January 2004 Stomatopoda (Crustacea: Hoplocarida) from the Shallow, Inshore Waters of the Northern Gulf of Mexico (Apalachicola River, Florida to Port Aransas, Texas) John M. Foster University of Southern Mississippi, [email protected] Brent P. Thoma University of Southern Mississippi Richard W. Heard University of Southern Mississippi, [email protected] Follow this and additional works at: https://aquila.usm.edu/gcr Part of the Marine Biology Commons Recommended Citation Foster, J. M., B. P. Thoma and R. W. Heard. 2004. Stomatopoda (Crustacea: Hoplocarida) from the Shallow, Inshore Waters of the Northern Gulf of Mexico (Apalachicola River, Florida to Port Aransas, Texas). Gulf and Caribbean Research 16 (1): 49-58. Retrieved from https://aquila.usm.edu/gcr/vol16/iss1/7 DOI: https://doi.org/10.18785/gcr.1601.07 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Gulf and Caribbean Research Vol 16, 49–58, 2004 Manuscript received December 15, 2003; accepted January 28, 2004 STOMATOPODA (CRUSTACEA: HOPLOCARIDA) FROM THE SHALLOW, INSHORE WATERS OF THE NORTHERN GULF OF MEXICO (APALACHICOLA RIVER, FLORIDA TO PORT ARANSAS, TEXAS) John M. Foster, Brent P. Thoma, and Richard W. Heard Department of Coastal Sciences, The University of Southern Mississippi, 703 East Beach Drive, Ocean Springs, Mississippi 39564, E-mail [email protected] (JMF), [email protected] (BPT), [email protected] (RWH) ABSTRACT Six species representing the order Stomatopoda are reported from the shallow, inshore waters (passes, bays, and estuaries) of the northern Gulf of Mexico limited to a depth of 10 m or less, and by the Apalachicola River (Florida) in the east and Port Aransas (Texas) in the west. -

Adobe Photoshop
Peer-Reviewed Journal Tracking and Analyzing Disease Trends pages 167–336 EDITOR-IN-CHIEF D. Peter Drotman Managing Senior Editor EDITORIAL BOARD Polyxeni Potter, Atlanta, Georgia, USA Dennis Alexander, Addlestone Surrey, United Kingdom Senior Associate Editor Timothy Barrett, Atlanta, GA, USA Brian W.J. Mahy, Bury St. Edmunds, Suffolk, UK Barry J. Beaty, Ft. Collins, Colorado, USA Associate Editors Martin J. Blaser, New York, New York, USA Paul Arguin, Atlanta, Georgia, USA Christopher Braden, Atlanta, GA, USA Charles Ben Beard, Ft. Collins, Colorado, USA Carolyn Bridges, Atlanta, GA, USA Ermias Belay, Atlanta, GA, USA Arturo Casadevall, New York, New York, USA David Bell, Atlanta, Georgia, USA Kenneth C. Castro, Atlanta, Georgia, USA Corrie Brown, Athens, Georgia, USA Louisa Chapman, Atlanta, GA, USA Charles H. Calisher, Ft. Collins, Colorado, USA Thomas Cleary, Houston, Texas, USA Michel Drancourt, Marseille, France Vincent Deubel, Shanghai, China Paul V. Effl er, Perth, Australia Ed Eitzen, Washington, DC, USA David Freedman, Birmingham, AL, USA Daniel Feikin, Baltimore, MD, USA Peter Gerner-Smidt, Atlanta, GA, USA Kathleen Gensheimer, Cambridge, MA, USA Stephen Hadler, Atlanta, GA, USA Duane J. Gubler, Singapore Nina Marano, Atlanta, Georgia, USA Richard L. Guerrant, Charlottesville, Virginia, USA Martin I. Meltzer, Atlanta, Georgia, USA Scott Halstead, Arlington, Virginia, USA David Morens, Bethesda, Maryland, USA David L. Heymann, London, UK J. Glenn Morris, Gainesville, Florida, USA Charles King, Cleveland, Ohio, USA Patrice Nordmann, Paris, France Keith Klugman, Atlanta, Georgia, USA Tanja Popovic, Atlanta, Georgia, USA Takeshi Kurata, Tokyo, Japan Didier Raoult, Marseille, France S.K. Lam, Kuala Lumpur, Malaysia Pierre Rollin, Atlanta, Georgia, USA Stuart Levy, Boston, Massachusetts, USA Ronald M.