Options for the Biological Control of Vespula Wasps in New Zealand
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
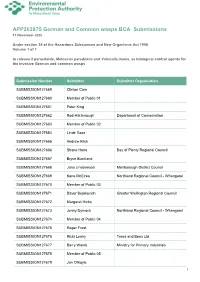
APP203875 Submissions Compilation.Pdf
APP203875 German and Common wasps BCA Submissions 11 November 2020 Under section 34 of the Hazardous Substances and New Organisms Act 1996 Volume 1 of 1 to release 2 parasitoids, Metoecus paradoxus and Volucella inanis, as biological control agents for the invasive German and common wasps Submission Number Submitter Submitter Organisation SUBMISSION127659 Clinton Care SUBMISSION127660 Member of Public 01 SUBMISSION127661 Peter King SUBMISSION127662 Rod Hitchmough Department of Conservation SUBMISSION127663 Member of Public 02 SUBMISSION127664 Linde Rose SUBMISSION127665 Andrew Blick SUBMISSION127666 Shane Hona Bay of Plenty Regional Council SUBMISSION127667 Bryce Buckland SUBMISSION127668 Jono Underwood Marlborough District Council SUBMISSION127669 Kane McElrea Northland Regional Council - Whangarei SUBMISSION127670 Member of Public 03 SUBMISSION127671 Davor Bejakovich Greater Wellington Regional Council SUBMISSION127672 Margaret Hicks SUBMISSION127673 Jenny Dymock Northland Regional Council - Whangarei SUBMISSION127674 Member of Public 04 SUBMISSION127675 Roger Frost SUBMISSION127676 Ricki Leahy Trees and Bees Ltd SUBMISSION127677 Barry Wards Ministry for Primary Industries SUBMISSION127678 Member of Public 05 SUBMISSION127679 Jan O'Boyle 1 Submission Number Submitter Submitter Organisation Apiculture New Zealand Science and SUBMISSION127680 Sue Carter Research Focus Group SUBMISSION127681 Andrea Dorn SUBMISSION127682 Benita Wakefield Te Rūnanga o Ngāi Tahu SUBMISSION127683 Emma Edney-Browne Auckland Council SUBMISSION127684 David Hunter -

White Admiral Newsletter
W h i t e A d m i r a l Newsletter 88 Summer 2014 Suffolk Naturalists’ Society C o n te n t s E d i t or i a l Ben Heather 1 Another new fungus for Suffolk Neil Mahler 2 The battle continues…the fight Matt Holden 4 against invasive alien plants in the Stour Valley! Nesting Materials Richard Stewart 8 How you can help monitor Suffolk’s Su e H o ot on 8 b a t s ? Records please! Rosemary Leaf Beetle Ben Heather 10 Stratiomys longicornis – a fly a long Peter Vincent 11 way from home! Periglacial Landforms in Breckland Caroline Markham 13 Are some roadside plants on the Dr. Anne Kell and 15 verge of extinction? Dennis Kell Where has all the road kill gone? Tom Langton 21 Back on the Hopper Trail in 2013 Colin Lucas & 22 Tricia Taylor Volucella zonaria – an impressive Peter Vincent 24 b e a s t Suffolk Show Wildlife H a w k H on e y 27 Suffolk’s Nature Strategy Nick Collinson 29 Species ‘Re - introductions’ Nick Miller 32 ISSN 0959-8537 Published by the Suffolk Naturalists’ Society c/o Ipswich Museum, High Street, Ipswich, Suffolk IP1 3QH Registered Charity No. 206084 © Suffolk Naturalists’ Society Front cover: Alder spittle bug - Aphrophora alni by Ben Heather Newsletter 88 - Summer 2014 Thank you to all those who have contributed to this full issue of the White Admiral newsletter. This issue covers a wide range of topics from roadside verges to an observation on the lack of roadkill on our roads. -

Invasive Alien Wasps in South Africa: Target Species and the Threats They Pose
About the project: What can you do to help? The Invasive Wasps Project is a research We are still pinpointing the exact distribution project with both eradication/infestation of both wasps in South Africa. If you see either control and communications components. of these wasps, please: The IWP project is managed by a consortium - Provide us with clear, close-up of researchers from the following institutions: photographs Stellenbosch University, the South African National Biodiversity Institute, the University - Provide us with locality information of Cape Town, the Agricultural Research – any landmarks or GPS co-ordinates Council, CapeNature, and Iziko Museums. will help us locate the wasps The project is researching the feasibility of - Provide us with details of Vespula eradicating both invasive wasps from South germanica nests or large (larger than Africa or, failing this, investigating ways to A5 paper size) Polistes dominula prevent their spread to new areas outside nests. their current, limited distribution. This is - Give us your contact details in case Invasive Alien necessary to avoid future human injury, we need to follow up and large scale biodiversity and economic Wasps in impacts. target species and South Africa: the threats they pose Please use caution when approaching any wasp species. Invasive Wasp Project Contact Details Ms Carol Poole: Administrative Coordinator E-mail: [email protected] Tel: 021 799 8652 Dr Ruan Veldtman: Research Coordinator E-mail: [email protected] Tel: 021 808 9441 Ms Karla Haupt: MSc student – Vespula germanica E-mail: [email protected] Mr Pc Benadé: MSc student – Polistes dominula E-mail: [email protected] Ms Carolien van Zyl: PhD student – Nest observations E-mail: [email protected] May 2013 The South African National Biodiversity Institute (SANBI) is mandated to conserve South Africa’s rich biodiversity. -

Read the Decision
Decision Date 5 February 2021 Application number APP203875 Application type To import for release and/or release from containment any new organism under section 34 of the Hazardous Substances and New Organisms Act 1996 Applicant Tasman District Council Date of hearing/consideration 17 December 2020 Date Application received 14 September 2020 Considered by A decision-making committee of the Environmental Protection Authority (the Committee)1 Dr Nick Roskruge (Chair) Dr John Taylor Mr Peter Cressey Purpose of the application To import and release two parasitoids, Metoecus paradoxus and Volucella inanis as biological control agents for the invasive German and common wasps (Vespula germanica and V. vulgaris). New organism approved Metoecus paradoxus Linnaeus 1761 Volucella inanis Linnaeus 1758 1 The Committee referred to in this decision is the subcommittee that has made the decision on this application under delegated authority in accordance with section 18A of the Act. Decision APP203875 Summary of decision 1. Application APP203875 to import and release two parasitoids, Metoecus paradoxus and Volucella inanis, as biological control agents (BCAs) for the invasive German and common wasps (Vespula germanica and V. vulgaris), in New Zealand, was lodged under section 34 of the Hazardous Substances and New Organisms Act 1996 (the Act). 2. The application was considered in accordance with the relevant provisions of the Act and of the HSNO (Methodology) Order 1998 (the Methodology). 3. The Committee has approved the application in accordance with section 38(1)(a) of the Act. Application and consideration process 4. The application was formally received on 14 September 2020. 5. The applicant, Tasman District Council, applied to the Environmental Protection Authority (EPA) to import and release two parasitoids, Metoecus paradoxus and Volucella inanis, as BCAs for the invasive German and common wasps (Vespula germanica and V. -

Acoustic Communication in the Nocturnal Lepidoptera
Chapter 6 Acoustic Communication in the Nocturnal Lepidoptera Michael D. Greenfield Abstract Pair formation in moths typically involves pheromones, but some pyra- loid and noctuoid species use sound in mating communication. The signals are generally ultrasound, broadcast by males, and function in courtship. Long-range advertisement songs also occur which exhibit high convergence with commu- nication in other acoustic species such as orthopterans and anurans. Tympanal hearing with sensitivity to ultrasound in the context of bat avoidance behavior is widespread in the Lepidoptera, and phylogenetic inference indicates that such perception preceded the evolution of song. This sequence suggests that male song originated via the sensory bias mechanism, but the trajectory by which ances- tral defensive behavior in females—negative responses to bat echolocation sig- nals—may have evolved toward positive responses to male song remains unclear. Analyses of various species offer some insight to this improbable transition, and to the general process by which signals may evolve via the sensory bias mechanism. 6.1 Introduction The acoustic world of Lepidoptera remained for humans largely unknown, and this for good reason: It takes place mostly in the middle- to high-ultrasound fre- quency range, well beyond our sensitivity range. Thus, the discovery and detailed study of acoustically communicating moths came about only with the use of electronic instruments sensitive to these sound frequencies. Such equipment was invented following the 1930s, and instruments that could be readily applied in the field were only available since the 1980s. But the application of such equipment M. D. Greenfield (*) Institut de recherche sur la biologie de l’insecte (IRBI), CNRS UMR 7261, Parc de Grandmont, Université François Rabelais de Tours, 37200 Tours, France e-mail: [email protected] B. -

2017 City of York Biodiversity Action Plan
CITY OF YORK Local Biodiversity Action Plan 2017 City of York Local Biodiversity Action Plan - Executive Summary What is biodiversity and why is it important? Biodiversity is the variety of all species of plant and animal life on earth, and the places in which they live. Biodiversity has its own intrinsic value but is also provides us with a wide range of essential goods and services such as such as food, fresh water and clean air, natural flood and climate regulation and pollination of crops, but also less obvious services such as benefits to our health and wellbeing and providing a sense of place. We are experiencing global declines in biodiversity, and the goods and services which it provides are consistently undervalued. Efforts to protect and enhance biodiversity need to be significantly increased. The Biodiversity of the City of York The City of York area is a special place not only for its history, buildings and archaeology but also for its wildlife. York Minister is an 800 year old jewel in the historical crown of the city, but we also have our natural gems as well. York supports species and habitats which are of national, regional and local conservation importance including the endangered Tansy Beetle which until 2014 was known only to occur along stretches of the River Ouse around York and Selby; ancient flood meadows of which c.9-10% of the national resource occurs in York; populations of Otters and Water Voles on the River Ouse, River Foss and their tributaries; the country’s most northerly example of extensive lowland heath at Strensall Common; and internationally important populations of wetland birds in the Lower Derwent Valley. -

Natural History of Lepidoptera Associated with Bird Nests in Mid-Wales
Entomologist’s Rec. J. Var. 130 (2018) 249 NATURAL HISTORY OF LEPIDOPTERA ASSOCIATED WITH BIRD NESTS IN MID-WALES D. H. B OYES Bridge Cottage, Middletown, Welshpool, Powys, SY21 8DG. (E-mail: [email protected]) Abstract Bird nests can support diverse communities of invertebrates, including moths (Lepidoptera). However, the understanding of the natural history of these species is incomplete. For this study, 224 nests, from 16 bird species, were collected and the adult moths that emerged were recorded. The majority of nests contained moths, with 4,657 individuals of ten species recorded. Observations are made on the natural history of each species and some novel findings are reported. The absence of certain species is discussed. To gain deeper insights into the life histories of these species, it would be useful to document the feeding habits of the larvae in isolation. Keywords : Commensal, detritivore, fleas, moths, Tineidae. Introduction Bird nests represent concentrated pockets of organic resources (including dead plant matter, feathers, faeces and other detritus) and can support a diverse invertebrate fauna. A global checklist compiled by Hicks (1959; 1962; 1971) lists eighteen insect orders associated with bird nests, and a study in England identified over 120 insect species, spanning eight orders (Woodroffe, 1953). Moths are particularly frequent occupants of bird nests, but large gaps in knowledge and some misapprehensions remain. For example, Tineola bisselliella (Hummel, 1823) was widely thought to infest human habitations via bird nests, which acted as natural population reservoirs. However, it has recently been discovered that this non-native species seldom occurs in rural bird nests and can be regarded as wholly synanthropic in Europe, where it was introduced from Africa around the turn of the 19th century (Plarre & Krüger- Carstensen, 2011; Plarre, 2014). -

Museum of Natural History
p m r- r-' ME FYF-11 - - T r r.- 1. 4,6*. of the FLORIDA MUSEUM OF NATURAL HISTORY THE COMPARATIVE ECOLOGY OF BOBCAT, BLACK BEAR, AND FLORIDA PANTHER IN SOUTH FLORIDA David Steffen Maehr Volume 40, No. 1, pf 1-176 1997 == 46 1ms 34 i " 4 '· 0?1~ I. Al' Ai: *'%, R' I.' I / Em/-.Ail-%- .1/9" . -_____- UNIVERSITY OF FLORIDA GAINESVILLE Numbers of the BULLETIN OF THE FLORIDA MUSEUM OF NATURAL HISTORY am published at irregular intervals Volumes contain about 300 pages and are not necessarily completed in any one calendar year. JOHN F. EISENBERG, EDITOR RICHARD FRANZ CO-EDIWR RHODA J. BRYANT, A£ANAGING EMOR Communications concerning purchase or exchange of the publications and all manuscripts should be addressed to: Managing Editor. Bulletin; Florida Museum of Natural Histoty, University of Florida P. O. Box 117800, Gainesville FL 32611-7800; US.A This journal is printed on recycled paper. ISSN: 0071-6154 CODEN: BF 5BAS Publication date: October 1, 1997 Price: $ 10.00 Frontispiece: Female Florida panther #32 treed by hounds in a laurel oak at the site of her first capture on the Florida Panther National Wildlife Refuge in central Collier County, 3 February 1989. Photograph by David S. Maehr. THE COMPARATIVE ECOLOGY OF BOBCAT, BLACK BEAR, AND FLORIDA PANTHER IN SOUTH FLORIDA David Steffen Maehri ABSTRACT Comparisons of food habits, habitat use, and movements revealed a low probability for competitive interactions among bobcat (Lynx ndia). Florida panther (Puma concotor cooi 1 and black bear (Urns amencanus) in South Florida. All three species preferred upland forests but ©onsumed different foods and utilized the landscape in ways that resulted in ecological separation. -

Yorkhill Green Spaces Wildlife Species List
Yorkhill Green Spaces Wildlife Species List April 2021 update Yorkhill Green Spaces Species list Draft list of animals, plants, fungi, mosses and lichens recorded from Yorkhill, Glasgow. Main sites: Yorkhill Park, Overnewton Park and Kelvinhaugh Park (AKA Cherry Park). Other recorded sites: bank of River Kelvin at Bunhouse Rd/ Old Dumbarton Rd, Clyde Expressway path, casual records from streets and gardens in Yorkhill. Species total: 711 Vertebrates: Amhibians:1, Birds: 57, Fish: 7, Mammals (wild): 15 Invertebrates: Amphipods: 1, Ants: 3, Bees: 26, Beetles: 21, Butterflies: 11, Caddisflies: 2, Centipedes: 3, Earthworms: 2, Earwig: 1, Flatworms: 1, Flies: 61, Grasshoppers: 1, Harvestmen: 2, Lacewings: 2, Mayflies: 2, Mites: 4, Millipedes: 3, Moths: 149, True bugs: 13, Slugs & snails: 21, Spiders: 14, Springtails: 2, Wasps: 13, Woodlice: 5 Plants: Flowering plants: 174, Ferns: 5, Grasses: 13, Horsetail: 1, Liverworts: 7, Mosses:17, Trees: 19 Fungi and lichens: Fungi: 24, Lichens: 10 Conservation Status: NameSBL - Scottish Biodiversity List Priority Species Birds of Conservation Concern - Red List, Amber List Last Common name Species Taxon Record Common toad Bufo bufo amphiban 2012 Australian landhopper Arcitalitrus dorrieni amphipod 2021 Black garden ant Lasius niger ant 2020 Red ant Myrmica rubra ant 2021 Red ant Myrmica ruginodis ant 2014 Buff-tailed bumblebee Bombus terrestris bee 2021 Garden bumblebee Bombus hortorum bee 2020 Tree bumblebee Bombus hypnorum bee 2021 Heath bumblebee Bombus jonellus bee 2020 Red-tailed bumblebee Bombus -

Investigation of Matrilineal Relationships Via Mitochondrial
Eastern Illinois University The Keep Masters Theses Student Theses & Publications 2003 Investigation of Matrilineal Relationships via Mitochondrial DNA in the Southeastern Yellowjacket (Vespula squamosa) Anthony Deets Eastern Illinois University This research is a product of the graduate program in Biological Sciences at Eastern Illinois University. Find out more about the program. Recommended Citation Deets, Anthony, "Investigation of Matrilineal Relationships via Mitochondrial DNA in the Southeastern Yellowjacket (Vespula squamosa)" (2003). Masters Theses. 1488. https://thekeep.eiu.edu/theses/1488 This is brought to you for free and open access by the Student Theses & Publications at The Keep. It has been accepted for inclusion in Masters Theses by an authorized administrator of The Keep. For more information, please contact [email protected]. THESIS/FIELD EXPERIENCE PAPER REPRODUCTION CERTIFICATE TO: Graduate Degree Candidates (who have written formal theses) SUBJECT: Permission to Reproduce Theses The University Library is receiving a number of request from other institutions asking permission to reproduce dissertations for inclusion in their library holdings. Although no copyright laws are involved, we feel that professional courtesy demands that permission be obtained from the author before we allow these to be copied. PLEASE SIGN ONE OF THE FOLLOWING STATEMENTS: Booth Library of Eastern Illinois University has my permission to lend my thesis to a reputable college or university for the purpose of copying it for inclusion in that institution's -

Appendix 1 Appendix 2
Oikos OIK-07259 de Manincor, N., Hautekeete, N., Piquot, Y., Schatz, B., Vanappelghem, C. and Massol, F. 2020. Does phenology explain plant–pollinator interactions at different latitudes? An assessment of its explanatory power in plant–hoverfly networks in French calcareous grasslands. – Oikos doi: 10.1111/oik.07259 Appendix 1 The following Supplementary material is available for this article: Figure A1. Sites location in France. Figure A2. Block clustering provided by LBM in the site of Falaises (FAL, Normandie), overlaid on a heatmap of species phenology overlap. Figure A3. Block clustering provided by LBM in the site of Bois de Fontaret (BF, Occitanie), overlaid on a heatmap of species phenology overlap. Figure A4. Block clustering provided by LBM in the site of Larris (LAR, Hauts-de-France), overlaid on a heatmap of species phenology overlap. Figure A5. Block clustering provided by LBM in the site of Riez (R, Hauts-de-France), overlaid on a heatmap of species phenology overlap. Table A1. Table of transformed plant abundances. Table A2. Table of hoverfly and plant species names and abbreviations used in the LBM Figures. Table A3. Table of model accuracy. Appendix 2 Appendix 2.1. Script modularity and latent block model analysis (LBM). Appendix 2.2. Model code. Appendix 2.3. Model script for the 16 models. 1 Appendix 1 Figure A1. Site location in France: in blue the French départements Pas-de-Calais and Somme (Hauts-de-France region), in green the départements Eure and Seine Maritime (Normandie region), in orange the départment Gard (Occitanie region). The six sites correspond to the red dots (with the sites of Fourches and Bois de Fontaret represented by the same dot due to their closeness). -

Wasps: Australian Paper Wasp (Polistes Humilis), Asian Paper Wasp (Polistes Chinensis), Common Wasp (Vespula Vulgaris) and German Wasp (Vespula Germanica)
Biosecurity series – animal factsheet Wasps: Australian paper wasp (Polistes humilis), Asian paper wasp (Polistes chinensis), common wasp (Vespula vulgaris) and German wasp (Vespula germanica) Advisory animal Eradication Progressive containment Sustained control Site-led Stop Australian, Asian, common and German wasps from damaging high value biodiversity sites. WHY WASPS ARE PESTS Production threat Environmental threat Public threat Four species of wasps in the Waikato region are considered pests, the Australian paper wasp, Asian paper wasp, common wasp and German wasp. German and common wasps pose the greatest risk to human health. They also attack beehives and prey on native insects. The common wasp IDENTIFYING FEATURES and the German wasp inhabit agricultural areas, native forests, planted Common and German wasps are slightly larger than forests, scrub/shrublands and urban areas where they nest underground honey bees, with distinctive black and yellow stripes. and in cavities in trees and buildings. Paper wasps are generally smaller with less yellow in Asian and Australian paper wasps are far less aggressive than German and their markings. common wasps, but they also prey on insects and chew weatherboards. The Asian paper wasp is larger than the Australian paper wasp. It Common and German wasps • Generally 12 to17mm long, although queens are arrived in New Zealand in the late 1970s and by 1995 was widespread larger. throughout the Upper North Island. It thrives in lowland open habitats such as shrublands, swamps and salt marshes but will often build nests on • Slightly bigger than a honey bee with smooth houses or other buildings as well as nesting in trees or bushes.