Population Structure, Growth and Production Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AEBR 114 Review of Factors Affecting the Abundance of Toheroa Paphies
Review of factors affecting the abundance of toheroa (Paphies ventricosa) New Zealand Aquatic Environment and Biodiversity Report No. 114 J.R. Williams, C. Sim-Smith, C. Paterson. ISSN 1179-6480 (online) ISBN 978-0-478-41468-4 (online) June 2013 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-resources/publications.aspx http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright - Ministry for Primary Industries TABLE OF CONTENTS EXECUTIVE SUMMARY ....................................................................................................... 1 1. INTRODUCTION ............................................................................................................ 2 2. METHODS ....................................................................................................................... 3 3. TIME SERIES OF ABUNDANCE .................................................................................. 3 3.1 Northland region beaches .......................................................................................... 3 3.2 Wellington region beaches ........................................................................................ 4 3.3 Southland region beaches ......................................................................................... -

Fossil Flora and Fauna of Bosnia and Herzegovina D Ela
FOSSIL FLORA AND FAUNA OF BOSNIA AND HERZEGOVINA D ELA Odjeljenje tehničkih nauka Knjiga 10/1 FOSILNA FLORA I FAUNA BOSNE I HERCEGOVINE Ivan Soklić DOI: 10.5644/D2019.89 MONOGRAPHS VOLUME LXXXIX Department of Technical Sciences Volume 10/1 FOSSIL FLORA AND FAUNA OF BOSNIA AND HERZEGOVINA Ivan Soklić Ivan Soklić – Fossil Flora and Fauna of Bosnia and Herzegovina Original title: Fosilna flora i fauna Bosne i Hercegovine, Sarajevo, Akademija nauka i umjetnosti Bosne i Hercegovine, 2001. Publisher Academy of Sciences and Arts of Bosnia and Herzegovina For the Publisher Academician Miloš Trifković Reviewers Dragoljub B. Đorđević Ivan Markešić Editor Enver Mandžić Translation Amra Gadžo Proofreading Amra Gadžo Correction Sabina Vejzagić DTP Zoran Buletić Print Dobra knjiga Sarajevo Circulation 200 Sarajevo 2019 CIP - Katalogizacija u publikaciji Nacionalna i univerzitetska biblioteka Bosne i Hercegovine, Sarajevo 57.07(497.6) SOKLIĆ, Ivan Fossil flora and fauna of Bosnia and Herzegovina / Ivan Soklić ; [translation Amra Gadžo]. - Sarajevo : Academy of Sciences and Arts of Bosnia and Herzegovina = Akademija nauka i umjetnosti Bosne i Hercegovine, 2019. - 861 str. : ilustr. ; 25 cm. - (Monographs / Academy of Sciences and Arts of Bosnia and Herzegovina ; vol. 89. Department of Technical Sciences ; vol. 10/1) Prijevod djela: Fosilna flora i fauna Bosne i Hercegovine. - Na spor. nasl. str.: Fosilna flora i fauna Bosne i Hercegovine. - Bibliografija: str. 711-740. - Registri. ISBN 9958-501-11-2 COBISS/BIH-ID 8839174 CONTENTS FOREWORD ........................................................................................................... -

Embryonic & Larval Development Gadomski.Pdf (646.7
Journal of Molluscan Studies Advance Access published 16 February 2015 Journal of The Malacological Society of London Molluscan Studies Journal of Molluscan Studies (2015) 1–9. doi:10.1093/mollus/eyv001 Embryonic and larval development of the New Zealand bivalve Paphies ventricosa Gray, 1843 (Veneroida: Mesodesmatidae) at a range of temperatures Kendall Gadomski1, Henrik Moller2, Michael Beentjes3 and Miles Lamare1 1Department of Marine Science, University of Otago, Dunedin, New Zealand; 2Centre for Sustainability (CSAFE), University of Otago, Dunedin, New Zealand; and 3National Institute of Water and Atmospheric Research, 38 Harrow Street, Dunedin, New Zeland Correspondence: M. Lamare; e-mail: [email protected] (Received 3 June 2014; accepted 14 December 2014) ABSTRACT Paphies ventricosa is a large (up to 150 mm shell length) surf clam endemic to New Zealand, with a geo- graphically patchy distribution. Using scanning electron microscopy and light microscopy, its fertiliza- tion, embryonic and larval development were observed at three culturing temperatures (12, 16 and 20 8C). The progress of development follows that previously described for the family Mesodesmatidae, with P. ventricosa having a small egg (63–70 mm), with a 83–102 mm trochophore stage observed at 15 h, and a 100 mm D-veliger larva observed at 22 h at 12 and 168C, and 37 h at 20 8C. At 20 8C, the pediveliger larval stage was reached by 31 d. While the morphology of the embryonic and larval stages of P. ventricosa is typical for bivalves, we show that in this species the shell field invagination occurs in the gastrula stage and that the expansion of the dorsal shell field occurs during gastrulation, with the early trochophore having a well-developed shell field that has a clearly defined axial line between the two shell lobes. -

The Zebra Mussel in Europe“
Chapter 2 NeogeNe dreisseNids iN CeNtral europe: evolutioNary shifts aNd diversity ChaNges Mathias Harzhauser and Oleg Mandic This chapter was originally published in the book „The Zebra Mussel in Europe“. The copy attached is provided by Margraf Publishers GmbH for the author‘s benefit and for the benefit of the author‘s institution for non-commercial research and educational use. All other uses, reproduction and distribution are prohibited and require a written permission by the publisher. G. van der Velde, S. Rajagopal & A. bij de Vaate de bij A. & Rajagopal S. Velde, der van G. The Zebra Mussel in Europe THE ZEBRA MUSSEL IN EUROPE Europe in Mussel Zebra The Edited by Gerard van der Velde, Sanjeevi Rajagopal & Abraham bij de Vaate The Zebra mussel (Dreissena polymorpha) is one of world’s most successful invasive species. Originating from the Ponto-Caspian region, it spread all over Europe and crossed over to North America via the ocean. Wherever it has spread, it made its presence felt with tremendous ecological and economic impact. To make matters worse, the species is still expanding its geographical range. Although there is a stream of information concerning the zebra mussel and its relatives, a recent up-to-date book summarizing the newest information on the zebra mussel was lacking. The present book is expected to fill this gap. It deals with edited by all aspects of the zebra mussel and some of its relatives, varying in its content from Gerard van der Velde taxonomy and phylogeny, to fossil and recent species, distribution and dispersal, genetics, food, growth and life history, ecology and ecological impact, endosymbionts, parasites, Sanjeevi Rajagopal predation, use as indicator for water quality and for water quality improvement and biofouling Abraham bij de Vaate and control. -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -
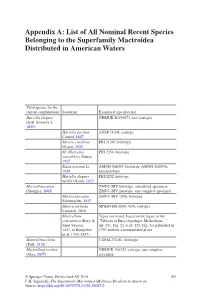
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Population Dynamics of the Argentinean Surf Clams Donax Hanleyanus and Mesodesma Mactroides from Open-Atlantic Beaches Off Argentina
Population dynamics of the Argentinean surf clams Donax hanleyanus and Mesodesma mactroides from open-Atlantic beaches off Argentina Populationsdynamik der Argentinischen Brandungsmuscheln Donax hanleyanus und Mesodesma mactroides offener Atlantikstrände vor Argentinien Marko Herrmann Dedicated to my family Marko Herrmann Alfred Wegener Institute for Polar and Marine Research (AWI) Section of Marine Animal Ecology P.O. Box 120161 D-27515 Bremerhaven (Germany) [email protected] Submitted for the degree Dr. rer. nat. of the Faculty 2 of Biology and Chemistry University of Bremen (Germany), October 2008 Reviewer and principal supervisor: Prof. Dr. Wolf E. Arntz 1 Reviewer and co-supervisor: Dr. Jürgen Laudien 1 External Reviewer: Dr. Pablo E. Penchaszadeh 2 1 Alfred Wegener Institute for Polar and Marine Research (AWI) Section of Marine Animal Ecology P.O. Box 120161 D-27515 Bremerhaven (Germany) 2 Director of the Ecology Section at the Museo Argentino de Ciencias Naturales (MACN) - Bernardino Rivadavia Av. Angel Gallardo 470, 3° piso lab. 80 C1405DJR Buenos Aires (Argentina) Contents 1 Summary .................................................................................................... 5 1.1 English Version ........................................................................................... 5 1.2 Deutsche Version ........................................................................................ 9 1.3 Versión Español ........................................................................................ 13 2 Introduction -

Historical Translocations by Māori May Explain the Distribution and Genetic
www.nature.com/scientificreports OPEN Historical translocations by Māori may explain the distribution and genetic structure of a threatened Received: 12 June 2018 Accepted: 6 November 2018 surf clam in Aotearoa (New Published: xx xx xxxx Zealand) Philip M. Ross 1, Matthew A. Knox2,3, Shade Smith4, Huhana Smith5, James Williams6 & Ian D. Hogg2,7 The population genetic structure of toheroa (Paphies ventricosa), an Aotearoa (New Zealand) endemic surf clam, was assessed to determine levels of inter-population connectivity and test hypotheses regarding life history, habitat distribution and connectivity in coastal vs. estuarine taxa. Ninety-eight toheroa from populations across the length of New Zealand were sequenced for the mitochondrial cytochrome c oxidase I gene with analyses suggesting a population genetic structure unique among New Zealand marine invertebrates. Toheroa genetic diversity was high in Te Ika-a Māui (the North Island of New Zealand) but completely lacking in the south of Te Waipounamu (the South Island), an indication of recent isolation. Changes in habitat availability, long distance dispersal events or translocation of toheroa to southern New Zealand by Māori could explain the observed geographic distribution of toheroa and their genetic diversity. Given that early-Māori and their ancestors, were adept at food cultivation and relocation, the toheroa translocation hypothesis is plausible and may explain the disjointed modern distribution of this species. Translocation would also explain the limited success in restoring what may in some cases be ecologically isolated populations located outside their natural distributions and preferred niches. Dispersal and connectivity among populations of marine organisms are strongly infuenced by a species’ life his- tory characteristics1. -

SURVEY of the LITERATURE on RECENT SHELLS from the RED SEA (Second Enlarged and Revised Edition)
TRITON 24 SEPTEMBER 2011 SUPPLEMENT 1 SURVEY OF THE LITERATURE ON RECENT SHELLS FROM THE RED SEA (second enlarged and revised edition) L.J. van Gemert *) Abstract: About 2,100 references are listed in the survey. Shells are being considered here as shell-bearing mollusks of the Gastropoda, Bivalvia and Scaphopoda. And the region covered is not only the Red Sea, but also the Gulf of Aden, including Somalia, and the Suez Canal, including Lessepsian species. Literature on fossils finds, especially from the Pliocene, Pleistocene and Holocene, is listed too. Introduction My interest in recent shells from the Red Sea dates from about 1996. Since then, I have been, now and then, trying to obtain information on this subject. Recently I decide to stop gathering information in a haphazard way and to do it more properly. This resulted in a survey of approximately 1,420 references (Van Gemert, 2010). Since then, this survey has been enlarged considerably and contains now approximately 2,100 references. They are presented here. Scope In principle every publication in which mollusks are reported to live or have lived in the Red Sea should be listed in the survey. This means that besides primary literature, i.e. articles in which researchers are reporting their finds for the first time, secondary and tertiary literature, i.e. reviews, monographs, books, etc are to be included too. These publications were written not only by a wide range of authors ranging from amateur shell collectors to profesional malacologists but also by people interested in other fields. This implies that not only malacological journals and books should be considered, but also publications from other fields or disciplines, such as environmental pollution, toxicology, parasitology, aquaculture, fisheries, biochemistry, biogeography, geology, sedimentology, ecology, archaeology, Egyptology and palaeontology, in which Red Sea shells are mentioned. -

AND MESODESMA ARCTATUM (CONRAD) of the NORTHWESTERN ATLANTIC JOHN DUNNING DAVIS University of New Hampshire, Durham
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Summer 1963 A STUDY OF THE ARCTIC WEDGE CLAMS, MESODESMA DEAURATUM (TURTON) AND MESODESMA ARCTATUM (CONRAD) OF THE NORTHWESTERN ATLANTIC JOHN DUNNING DAVIS University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation DAVIS, JOHN DUNNING, "A STUDY OF THE ARCTIC WEDGE CLAMS, MESODESMA DEAURATUM (TURTON) AND MESODESMA ARCTATUM (CONRAD) OF THE NORTHWESTERN ATLANTIC" (1963). Doctoral Dissertations. 2337. https://scholars.unh.edu/dissertation/2337 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. This dissertation has been 64—202 microfilmed exactly as received DAVIS, John Dunning, 1929— A STUDY OF THE ARCTIC WEDGE CLAMS, MESODESMA DEAURATUM (TURTON) AND MESODESMA ARCTATUM (CONRAD) OF THE NORTHWESTERN ATLANTIC. University of New Hampshire, Ph.D., 1963 University Microfilms, Inc., Ann Arbor, Michigan A STUDY OF THE ARCTIC WEDGE CLAMS, MESODESMA DEAURATUM (TURTCN) AND MESODESMA ARCTATUH (CONRAD) OF THE NORTHWESTERN ATLANTIC BY , JOHN D. DAVIS A. B., Bowdoin College, 19J?2 Ed. M . , Boston University, 195^4- M. S. University of New Hampshire, 1961 A THESIS Submitted to the University of New Hampshire In Partial Fulfillment of The Requirements for the Degree of Doctor of Philosophy Graduate School Department of Zoology June, 1963 This thesis has been examined and approved f t J Y ^ y " Y W t & e ’ v../‘ ACKNOWLEDGMENTS The conclusion of a work of this scope requires that the invaluable aid of numerous 'persons be recognized. -

(Mollusca: Bivalvia) from Singapore
OCCASIONAL MOLLUSCAN PAPERS 2010 2: 1 – 4. ISSN 1793-8708 (print edition) Date of Publication: 3rd August 2010 ISSN 1793-8716 (online edition) © molluscan.com ON THREE SPECIES OF MESODESMATIDAE (MOLLUSCA: BIVALVIA) FROM SINGAPORE S.-Y. Chan VBox 888313, Singapore 919191. Email: [email protected] ABSTRACT Three species of intertidal bivalves belonging to the family Mesodesmatidae (commonly known as “wedge clams”) have had confirmed records (Morris & Purchon, 1982; Tan & Woo, 2010) from Singapore and is diagnosable on the grounds of shell morphology. They are Davila plana, Paphies striata and Coecella horsfieldii. This article aims to present the local mesodesmatids pictorially with some descriptive and ecological notes. KEYWORDS Systematics, taxonomy, Mollusca, Bivalvia, Mesodesmatidae, Singapore. INTRODUCTION The forty or so species of equivalved Mesodesmatidae (Rosenberg, 1992) is a marine to estuarine clam family of thick shells with an internal ligament located in the center of the hinge teeth (Tan & Chou, 2000) sitting on a pit (Kilburn & Rippey, 1982; Wong, 2009). This family has close affinity to “surf clams” Mactridae (Rooij-Schuiling, 1977; Vongpanich 2000; Wong, 2009) in the superfamily Mactroidea (Vaught, 1989; Tan & Woo, 2010). Two Singaporean species (Paphies striata and Coecella horsfieldii) are found from the mid to high intertidal regions of sandy beaches, and empty shells are quite common among beach debris at the high water mark. These two larger species, however, seemed to have some commercial fishery importance (Rooij-Schuiling, 1972; Law, 1976; Morris & Purchon, 1981) in Singapore and are exploited in Fiji Islands, India and Japan (Poutiers, 1998). MATERIALS AND METHODS Three dead shells of mesodesmatids (Plate 1) found among beach debris, were picked from various locations along the stretch of East Coast Park from 2nd January 1994 to 5th June 2010. -

TUATUA (TUA) (Paphies Subtriangulata) Tuatua 1. FISHERY
TUATUA (TUA) TUATUA (TUA) (Paphies subtriangulata ) Tuatua TUA1A TUA9 TUA 1B TUA8 TUA 2 TUA 7 TUA 4 TUA3 TUA 5 1. FISHERY SUMMARY 1.1 Commercial fisheries Tuatua ( Paphies subtriangulata ) were introduced into the QMS on 1 October 2005. The fishing year runs from 1 October to 30 September, and commercial catches are measured in greenweight. QMA boundaries for tuatua were set the same as those established for FMAs, except for FMA 1 (the area between North Cape and Cape Runaway), which was divided into two QMAs, TUA 1A and TUA 1B, on either side of Te Arai Point (Pakiri Beach). The formerly specified historic commercial areas within TUA 1B (Papamoa domain to Maketu Beach, Bay of Plenty) and TUA 9 (i.e., Ninety Mile Beach, Hokianga Harbour to Maunganui Bluff, and specific areas between Maunganui Bluff to the North Head of the Kaipara Harbour) were revoked, and regulations were amended to remove the commercial daily catch limits for tuatua, which were no longer applicable. Commercial fishing was allowed to continue only in TUA 9 in the specified commercial area of the Kaipara Harbour entrance. A TACC of 43 t, which reflected the average of the reported landings taken from the Kaipara fishery between 1990–91 and 2003–04, was allocated to the TUA 9 stock in recognition that commercial tuatua fishing was constrained to the Kaipara Harbour entrance. There is no minimum legal size (MLS) for tuatua, although fishers probably favour large individuals. Tuatua are available for harvest year-round, so there is no apparent seasonality in the fishery.