Japanese Beetle Host Plant List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHESTNUT (CASTANEA Spp.) CULTIVAR EVALUATION for COMMERCIAL CHESTNUT PRODUCTION
CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE By Ana Maria Metaxas Approved: James Hill Craddock Jennifer Boyd Professor of Biological Sciences Assistant Professor of Biological and Environmental Sciences (Director of Thesis) (Committee Member) Gregory Reighard Jeffery Elwell Professor of Horticulture Dean, College of Arts and Sciences (Committee Member) A. Jerald Ainsworth Dean of the Graduate School CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE by Ana Maria Metaxas A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements for the Degree of Master of Science in Environmental Science May 2013 ii ABSTRACT Chestnut cultivars were evaluated for their commercial applicability under the environmental conditions in Hamilton County, TN at 35°13ꞌ 45ꞌꞌ N 85° 00ꞌ 03.97ꞌꞌ W elevation 230 meters. In 2003 and 2004, 534 trees were planted, representing 64 different cultivars, varieties, and species. Twenty trees from each of 20 different cultivars were planted as five-tree plots in a randomized complete block design in four blocks of 100 trees each, amounting to 400 trees. The remaining 44 chestnut cultivars, varieties, and species served as a germplasm collection. These were planted in guard rows surrounding the four blocks in completely randomized, single-tree plots. In the analysis, we investigated our collection predominantly with the aim to: 1) discover the degree of acclimation of grower- recommended cultivars to southeastern Tennessee climatic conditions and 2) ascertain the cultivars’ ability to survive in the area with Cryphonectria parasitica and other chestnut diseases and pests present. -

Guidelines for the Design and Construction Of
Guidelines for the Design and Construction of Stormwater Management Systems Developed by the New York City Department of Environmental Protection in consultation with the New York City Department of Buildings July 2012 Michael R. Bloomberg, Mayor Carter H. Strickland, Jr., Commissioner Cover: An extensive green roof system installed atop the NYC Department of Parks and Recreation’s (DPR) Five Borough Building on Randall’s Island. This modular system is one of six variations installed on the roof and covers 800 square feet, con- sisting of two-foot by two-foot trays with six inches of mineral soil and over 1,500 sedum plugs. DPR has installed 25 green roof systems covering over 29,000 square feet on the Five Borough Building rooftop to feature different types and depths of growing medium and plant selection. Dear Friends; The NYC Green Infrastructure Plan, released in September 2010, proposed an innovative ap- proach for cost-effective and sustainable stormwater management. A major part of this plan is our commitment to manage the equivalent of an inch of rainfall on ten percent of the impervious areas in combined sewer watersheds by 2030. To that end, DEP is prepared to spend $1.5 bil- lion to construct green infrastructure projects across the city. Yet public investment alone will not achieve our water quality goals, or our desired recreation and development opportunities. Some of the most cost-effective opportunities are represented by new construction and devel- opment, when stormwater source controls can be easily included in designs and built at a frac- tion of the cost of retrofitting existing buildings. -

5 Fagaceae Trees
CHAPTER 5 5 Fagaceae Trees Antoine Kremerl, Manuela Casasoli2,Teresa ~arreneche~,Catherine Bod6n2s1, Paul Sisco4,Thomas ~ubisiak~,Marta Scalfi6, Stefano Leonardi6,Erica ~akker~,Joukje ~uiteveld', Jeanne ~omero-Seversong, Kathiravetpillai Arumuganathanlo, Jeremy ~eror~',Caroline scotti-~aintagne", Guy Roussell, Maria Evangelista Bertocchil, Christian kxerl2,Ilga porth13, Fred ~ebard'~,Catherine clark15, John carlson16, Christophe Plomionl, Hans-Peter Koelewijn8, and Fiorella villani17 UMR Biodiversiti Genes & Communautis, INRA, 69 Route d'Arcachon, 33612 Cestas, France, e-mail: [email protected] Dipartimento di Biologia Vegetale, Universita "La Sapienza", Piazza A. Moro 5,00185 Rome, Italy Unite de Recherche sur les Especes Fruitikres et la Vigne, INRA, 71 Avenue Edouard Bourlaux, 33883 Villenave d'Ornon, France The American Chestnut Foundation, One Oak Plaza, Suite 308 Asheville, NC 28801, USA Southern Institute of Forest Genetics, USDA-Forest Service, 23332 Highway 67, Saucier, MS 39574-9344, USA Dipartimento di Scienze Ambientali, Universitk di Parma, Parco Area delle Scienze 1lIA, 43100 Parma, Italy Department of Ecology and Evolution, University of Chicago, 5801 South Ellis Avenue, Chicago, IL 60637, USA Alterra Wageningen UR, Centre for Ecosystem Studies, P.O. Box 47,6700 AA Wageningen, The Netherlands Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556, USA lo Flow Cytometry and Imaging Core Laboratory, Benaroya Research Institute at Virginia Mason, 1201 Ninth Avenue, Seattle, WA 98101, -

Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2
Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) by Boris C. Kondratieff, Paul A. Opler, Matthew C. Garhart, and Jason P. Schmidt C.P. Gillette Museum of Arthropod Diversity Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, Colorado 80523 March 15, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration (top to bottom): Widow Skimmer (Libellula luctuosa) [photo ©Robert Behrstock], Stonefly (Perlesta species) [photo © David H. Funk, White- lined Sphinx (Hyles lineata) [photo © Matthew C. Garhart] ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences, Colorado State University, Fort Collins, Colorado 80523 Copyrighted 2004 Table of Contents EXECUTIVE SUMMARY……………………………………………………………………………….…1 INTRODUCTION…………………………………………..…………………………………………….…3 OBJECTIVE………………………………………………………………………………………….………5 Site Descriptions………………………………………….. METHODS AND MATERIALS…………………………………………………………………………….5 RESULTS AND DISCUSSION………………………………………………………………………..…...11 Dragonflies………………………………………………………………………………….……..11 -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Mirabilis Jalapa: a Review of Ethno and Pharmacological Activities
Advancement in Medicinal Plant Research Vol. 9(1), pp. 1-10, January 2021 ISSN: 2354-2152 Review Mirabilis jalapa: A review of ethno and pharmacological activities Farjana Islam Liya, Mt. Farzana Yasmin, Nargis Sultana Chowdhury*, Tasnia Khasru Charu and Ismat Benta Fatema Department of Pharmacy, Manarat International University, Ashulia Model Town, Khagan, Ashulia, Dhaka, Bangladesh. Accepted 4 January, 2021 ABSTRACT Plants have been used for thousands of years to treat, prevent, and control a variety of diseases throughout the world. The initial benefits of using plant-derived medicine are that they are relatively safer than artificial alternatives. Mirabilis jalapa Linn. (Nyctaginaceae) is one of the plants that used for health care and medicinal purposes for several thousands of years. It is a perennial bushy herb promulgate by flowers or leaves, a native of America and commonly known as ‘four-o-clock’. It has traditionally been used in the treatment of gastrointestinal disorders, muscle pain, abdominal colic, and diarrhea in ancient Mexico, Japan, China and Brazil. The literature review revealed that M. jalapa is widely used as anti-oxidant, anti- inflammatory, anti-microbial, anti-diabetic, cytotoxic, antinociceptive, and several other’s medicines. In aerial parts of the M. jalapa, triterpene and flavonoids are found. Flowers mostly contain anthocyanins and flavonoids. Carbohydrate, resin and alkaloids are found in roots. Tricosan-12-one, n-hexacosanal, β- sitosterol, and tetracosanoic were isolated from the leaves of the M. jalapa. Seeds contain β-sitosterol, β- amyrin and β-sitosterol-D-glucoside. The presence of various bioactive compounds validates the whole plant for different medicinal practitioners. -
Species Lists
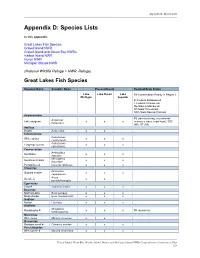
Appendix D: Species Lists Appendix D: Species Lists In this appendix: Great Lakes Fish Species Gravel Island NWR Gravel Island and Green Bay NWRs Harbor Island NWR Huron NWR Michigan Islands NWR (National Wildlife Refuge = NWR, Refuge) Great Lakes Fish Species Common Name Scientific Name Present/Absent Regional/State Status Lake Lake Huron Lake R3-Conservation Priority in Region 3 Michigan Superior E- Federal Endangered T-Federal Threatened SE-State Endangered ST-State Threatened SSC-State Special Concern Acipenseridae R3 (rare/declining, recreational/ Acipenser Lake sturgeon x x x economic value, tribal trust), SSC fulvescens (WI), ST (MI) Amiidae Bowfin Amia calva x x Catostomidae Catostomus White sucker x x x commersoni Catostomus Longnose sucker x x x catostomus Centrarchidae Ambloplites Rockbass x x x rupestris Micropterus Smallmouth bass x x x dolomieui Pumpkinseed Lepomis gibbosus x x x Clupeidae Dorosoma Gizzard shad # x x x cepedianum Alosa Alewife # x x pseudoharengus Cyprinidae Carp # Cyprinus Carpio x x x Esocidae Northern pike Esox Lucieus x x x Muskellunge Esox masquinongy x x x Gadidae Burbot Lota lota x x x Gobiidae Neogobius Round goby # x x x R3 (nuisance) melanostomus Moronidae White bass Morone chrysops x x Osmeridae Rainbow smelt # Osmerus mordax x x x Percichthyidae White perch # Morone americana x x x Gravel Island, Green Bay, Harbor Island, Huron, and Michigan Islands NWRs/Comprehensive Conservation Plan 221 Appendix D: Species Lists Common Name Scientific Name Present/Absent Regional/State Status Percidae R3 (rare/declining, -

ANNUALS for UTAH GARDENS Teresa A
ANNUALS FOR UTAH GARDENS Teresa A. Cerny Ornamental Horticulture Specialist Debbie Amundsen Davis County Horticulture Extension Agent Loralie Cox Cache County Horticulture Extension Agent September 2003 HG-2003/05 Annuals are plants that come up in the spring, reach maturity, flower, set seeds, then die all in one season. They provide eye-catching color to any flower bed and can be used as borders, fillers, or background plantings. There are several ways to find annual species that fit your landscape needs; referring to the All-American Selection program evaluations (http://www.all-americaselections.org), visiting botanical gardens to observe examples of annuals in the landscape, and looking through commercial seed catalogs are excellent places to find ideas. Most annuals are available in cell packs, flats, or individual pots. When buying plants, choose those that are well established but not pot bound. Tall spindly plants lack vigor and should be avoided. Instead look for plants with dark green foliage that are compact and free of insect and disease problems. These criteria are much more important than the flower number when choosing a plant. An abundance of foliage with few, if any flowers, is desirable. BED PREPARATION Avoid cultivating soil too early in the spring and during conditions that are too wet. Soil conditions can be determined by feeling the soil. If the soil forms a ball in your hand but crumbles easily, it is ideal. Cultivate the flower bed to a depth of 6-10 inches by turning the soil with a spade. Utah soils can always use extra organic matter such as grass clippings, leaves, compost, manure, peat, etc. -

Illinois Exotic Species List
Exotic Species in Illinois Descriptions for these exotic species in Illinois will be added to the Web page as time allows for their development. A name followed by an asterisk (*) indicates that a description for that species can currently be found on the Web site. This list does not currently name all of the exotic species in the state, but it does show many of them. It will be updated regularly with additional information. Microbes viral hemorrhagic septicemia Novirhabdovirus sp. West Nile virus Flavivirus sp. Zika virus Flavivirus sp. Fungi oak wilt Ceratocystis fagacearum chestnut blight Cryphonectria parasitica Dutch elm disease Ophiostoma novo-ulmi and Ophiostoma ulmi late blight Phytophthora infestans white-nose syndrome Pseudogymnoascus destructans butternut canker Sirococcus clavigignenti-juglandacearum Plants okra Abelmoschus esculentus velvet-leaf Abutilon theophrastii Amur maple* Acer ginnala Norway maple Acer platanoides sycamore maple Acer pseudoplatanus common yarrow* Achillea millefolium Japanese chaff flower Achyranthes japonica Russian knapweed Acroptilon repens climbing fumitory Adlumia fungosa jointed goat grass Aegilops cylindrica goutweed Aegopodium podagraria horse chestnut Aesculus hippocastanum fool’s parsley Aethusa cynapium crested wheat grass Agropyron cristatum wheat grass Agropyron desertorum corn cockle Agrostemma githago Rhode Island bent grass Agrostis capillaris tree-of-heaven* Ailanthus altissima slender hairgrass Aira caryophyllaea Geneva bugleweed Ajuga genevensis carpet bugleweed* Ajuga reptans mimosa -

Antiviral Potential of Plants Against Noroviruses
molecules Review Antiviral Potential of Plants against Noroviruses Jolanta Sarowska 1, Dorota Wojnicz 2,* , Agnieszka Jama-Kmiecik 1, Magdalena Frej-M ˛adrzak 1 and Irena Choroszy-Król 1 1 Department of Basic Sciences, Faculty of Health Sciences, Wroclaw Medical University, Chalubinskiego 4, 50-368 Wroclaw, Poland; [email protected] (J.S.); [email protected] (A.J.-K.); [email protected] (M.F.-M.); [email protected] (I.C.-K.) 2 Department of Biology and Medical Parasitology, Faculty of Medicine, Wroclaw Medical University, Mikulicza-Radeckiego 9, 50-345 Wroclaw, Poland * Correspondence: [email protected]; Tel.: +48-717-841-512 Abstract: Human noroviruses, which belong to the enterovirus family, are one of the most common etiological agents of food-borne diseases. In recent years, intensive research has been carried out regarding the antiviral activity of plant metabolites that could be used for the preservation of fresh food, because they are safer for consumption when compared to synthetic chemicals. Plant prepara- tions with proven antimicrobial activity differ in their chemical compositions, which significantly affects their biological activity. Our review aimed to present the results of research related to the characteristics, applicability, and mechanisms of the action of various plant-based preparations and metabolites against norovirus. New strategies to combat intestinal viruses are necessary, not only to ensure food safety and reduce infections in humans but also to lower the direct health costs associated with them. Citation: Sarowska, J.; Wojnicz, D.; Keywords: plant secondary metabolites; antiviral activity; food; noroviruses; MNV; FCV Jama-Kmiecik, A.; Frej-M ˛adrzak,M.; Choroszy-Król, I. -

Impact of Structured Water on Yield and Crop Quality of Radish (Raphanus Sativus L.)
Pol. J. Environ. Stud. Vol. 30, No. 5 (2021), 4895-4899 DOI: 10.15244/pjoes/131807 ONLINE PUBLICATION DATE: 2021-08-09 Short Communication Impact of Structured Water on Yield and Crop Quality of Radish (Raphanus sativus L.) Mirosław Gluch1, Jacek Gronek1, Jan Karch1, Andrzej Skrobiszewski1, Piotr Chohura2* 1Hydreset AG, Alti Luzeinerstrass 2, 7240 Küblis 6, Switzerland 2Department of Horticulture, The Faculty of Life Sciences and Technology, Wroclaw University of Environmental and Life Sciences; Grunwaldzki Sq. 24A, 50-363 Wrocław, Poland Received: 3 October 2020 Accepted: 18 December 2020 Abstract In the field experiment, radish was grown in the spring cycle and was watered with two types of water. Tap water (control) and structured water were used for irrigation. The use of structured water for radish irrigation contributed significantly to increase the yield in radish roots compared to the tap water. Radish watered with structured water contained more photosynthetic pigments and carotenoids compared to the control. The vitamin C content in radish leaves and roots was higher when tap water was used for irrigation, while nitrates level was lower after structured water using. The use of different types of water for radish watering had no significant effect on the concentration of minerals in the roots. The research confirmed the beneficial effect of structured water on the yield and the quality of the radish and revealed the possibility of using it for plants irrigation. Keywords: structured water, radish, Raphanus sativus, vegetable crops, yield Introduction yield, but also enhances productivity and fertility in some animal breedings. Nevertheless, the effect and Water is essential for all human beings due to its the role of structured water in agriculture and other significant and complex role in life processes such branches of industry are still need to be proven. -

Leaf Peltate Glandular Trichomes of Vernonia Galamensis Ssp
I Int. J. Plant Sd. 169(5):605-614. 2008. © 2008 by The University of Chicago. All rights reserved. 1058-5893/2008/16905-0002$15.00 DOt: 10.1086/533598 LEAF PELTATE GLANDULAR TRICHOMES OF VERNONIA GALAMENSIS SSP. GALAMENSIS VAR. ETHIOPICA GILBERT: DEVELOPMENT, ULTRASTRUCTURE, AND CHEMICAL COMPOSITION Françoise Favi,l Charles L. Cantrell,t Tadesse Mebrahtu, and Mark E. Kraemer *Agricultural Research Station, Virginia State University, Petersburg, Virginia 23805, U.S.A.; and tUSDA-ARS, Natural Products Utilization Research Unit, University, Mississippi 38677, U.S.A. Plants from the genus Vernonia produce a variety of flavonoids and bitter sesquiterpene tactones important for agriculture and human health. Leaf glandular trichornes of Vernonia galainensis ssp. galamensis var. ethiopica Gilbert (VGAE) were investigated for ultrastructural development and content composition because sesquiter- pene lactones that impart a bitter taste to the leaves have been associated with the presence of these glands. Trichome ultrastructure was examined using LM, SEM, and TEM. Glands were removed from the leaf surface, and the chemical composition of gland contents was determined using HPLC and high-resolution mass spec- trometry. Immature and mature 10-celled peltatc hiseriate glandular trichomes were present only at the abaxial side of the leaf. A large subcuticular space (head) developed from the most distal cell pair of the mature trichome and gradually filled with an osmiophillic substance. Mass spectrometry analysis revealed that the peltate trichome is a major source of prevernocistifolide-8-O-isobutyrate. This glaucolide-type sesquiterpene lactone was previously identified as a major constituent of the aerial parts of VGAL. Keywords: glaucolides, sesquiterpene lactones, Vernonia galanzensis, trichomes.