ICS) and Long-Acting Beta-Agonists (Labas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

Effects of Glycopyrronium on Lung Hyperinflation in COPD Patients Claudio M Sanguinetti1,2
Sanguinetti Multidisciplinary Respiratory Medicine 2014, 9:19 http://www.mrmjournal.com/content/9/1/19 REVIEW Open Access The lungs need to be deflated: effects of glycopyrronium on lung hyperinflation in COPD patients Claudio M Sanguinetti1,2 Abstract Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation caused by bronchial alterations, small airways disease and parenchymal destruction. In patients with COPD the structural and functional lung alterations can progress more or less rapidly from the initial small airways disease to an overt COPD where a severe expiratory flow limitation takes place. In these conditions, lung hyperinflation develops characterized by increase in functional residual capacity (FRC) and decrease in inspiratory capacity (IC). Thus, IC is an easy and reliable index to monitor lung hyperinflation and to assess the efficacy of bronchodilator drugs. When FRC increases, tidal volume (VT) is located in a more flatted upper part of the P –V curve of the respiratory system and respiratory muscles must sustain a greater elastic workload. Furthermore, due to inadequate time for expiration, there is a positive alveolar pressure at the end of expiration (PEEPi). This represents a further elastic workload for the inspiratory muscles. This impairment of ventilatory mechanics generates dyspnea that in most severely compromised patients occurs also for small efforts causing activity limitation and worst health-related quality of life (HRQoL). Due to these respiratory alterations, bronchodilators are the cornerstone of the long-term treatment of COPD in order to decrease airways resistances, lung hyperinflation and exacerbation rate, and improve patient’s symptoms, exercise tolerance and health status. -

Effects of Replacing Oxitropium with Tiotropium on Pulmonary Function in Patients with COPD: a Randomized Study
View metadata, citation and similar papers at core.ac.uk ARTICLE IN PRESS brought to you by CORE provided by Elsevier - Publisher Connector Respiratory Medicine (2007) 101, 476–480 Effects of replacing oxitropium with tiotropium on pulmonary function in patients with COPD: A randomized study Cristoforo IncorvaiaÃ, Gian Galeazzo Riario-Sforza, Chiara Pravettoni, Natale Dugnani, Fulvia Paterniti, Laura Pessina, Mario Fumagalli Unit of Pulmonary Rehabilitation, ICP Hospital, via Bignami 1, Viale Molise 69, Milan, Italy Received 3 February 2006; accepted 6 July 2006 KEYWORDS Summary COPD; Background: Inhaled bronchodilators are first line drugs in the treatment of chronic Pulmonary function; obstructive pulmonary disease (COPD). Tiotropium bromide is a recently introduced long- Drug treatment; acting anticholinergic agent able to reduce dyspnoea and COPD exacerbations and to Anticholinergics; improve pulmonary function and quality of life. We designed a study to compare the short- Tiotropium; term efficacy of tiotropium bromide with that of oxitropium bromide in improving Oxitropium pulmonary function in patients with COPD. Methods: Eighty patients were randomized either to continue oxitropium 800 mcg/day or to receive tiotropium 18 mcg/day. Seventy-six (39 in the tiotropium and 37 in the oxitropium group) completed the study. Plethysmography was performed at baseline and after 72 h in all patients. The changes in functional parameters in the two groups were compared by the Mann–Whitney U-test. Results: There were no differences between the two groups regarding age (72.5 vs. 74.2 years), male/female ratio (25/14 vs. 23/14) and pulmonary function at baseline. The changes in spirometric parameters were significantly greater in tiotropium- than in oxitropium-treated patients: mean forced expiratory volume in 1 s (FEV1) increased significantly by 15% vs. -
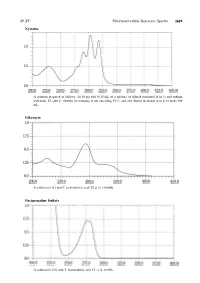
Nystatin Ofloxacin Orciprenaline Sulfate
JP XV Ultraviolet-visible Reference Spectra 1619 Nystatin A solution prepared as follows: To 10 mg add 50.25 mL of a mixture of diluted methanol (4 in 5) and sodium hydroxide TS (200:1), dissolve by warming at not exceeding 509C,andadddilutedmethanol(4in5)tomake500 mL. Ofloxacin Asolutionin0.1mol/LhydrochloricacidTS(1in150,000) Orciprenaline Sulfate A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) 1620 Ultraviolet-visible Reference Spectra JP XV Oxazolam A solution in ethanol (95) (1 in 100,000) Oxethazaine A solution in ethanol (95) (1 in 2500) Oxybuprocaine Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1621 Oxycodone Hydrochloride Hydrate An aqueous solution (1 in 10,000) Oxymetholone A solution prepared as follows: To 5 mL of a solution in methanol (1 in 5000) add 5 mL of sodium hydroxide-methanol TS and methanol to make 50 mL. Oxytetracycline Hydrochloride Asolutionin0.1mol/LhydrochloricacidTS(1in50,000) 1622 Ultraviolet-visible Reference Spectra JP XV Oxytocin An aqueous solution (1 in 2000) Penbutolol Sulfate A solution in methanol (1 in 10,000) Pentazocine A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) JP XV Ultraviolet-visible Reference Spectra 1623 Peplomycin Sulfate Asolutionpreparedasfollows:To4mgadd5mL of copper (II) sulfate TS, and dissolve in water to make 100 mL. Perphenazine 1 Asolutionin0.1mol/LhydrochloricacidTS(1in200,000) Perphenazine 2 A solution obtained by adding 10 mL of water to 10 mL of the solution for Perphenazine 1 1624 Ultraviolet-visible -

1. Generic Names Levosalbutamol 0.25G Ambroxol 7.5Mg
1. Generic Names Levosalbutamol 0.25g Ambroxol 7.5mg Guaiphenesin 12.5 mg 2. Qualitative and Quantitative Composition Each ml contains Levosalbutamol 0.25g Ambroxol 7.5mg Guaiphenesin 12.5 mg 3. Dosage form and strength Oral drop solution containing Levosalbutamol 0.25g, Ambroxol 7.5mg, Guaiphenesin 12.5 mg. 4. Clinical particulars 4.1 Therapeutic indication Kofarest-PD drops is indicated for the treatment of productive cough associated with bronchospasm in conditions such as bronchitis and bronchial asthma as well as all conditions associated with tenacious mucus, wheezing and chest congestion. 4.2 Posology and method of administration The usual recommended dose of KOFAREST-PD Drops in children is: 1-2 years age: 0.8 ml thrice a day 2-3 years age: 1.2 ml thrice a day. 4.3 Contraindication Kofarest-PD drops are contraindicated in patients with hypersensitivity to any ingredient of the formulation. 4.4 Special warnings and precautions for use While treating cough as a symptom, it is important to make every effort to determine and treat appropriately the underlying cause, such as a specific infection. Caution should be observed while prescribing Kofarest-PD drops to children with hypertension, cardiovascular disease, uncontrolled juvenile diabetes mellitus, hyperthyroidism, and seizures or in patients who are unusually hypersensitive to sympathomimetic amines. 4.5 Drug interactions Hypokalaemia with high doses of ß2 -agonists may result in increased susceptibility to digitalis induced cardiac arrhythmias. Hypokalaemia may be enhanced by concomitant administration of aminophylline or other xanthine, corticosteroids or by diuretic therapy. Other sympathomimetic bronchodilators or epinephrine should not be used concomitantly with salbutamol, since their combined effect on the cardiovascular system may be deleterious to the patient. -

Supplementary Materials 07/09/2017
SMART Adolescent manuscript: Supplementary materials 07/09/2017 Supplementary materials Methods Definition and calculation of severe exacerbations The first three studies conducted 10-12, defined severe exacerbations as the need for oral corticosteroid (OCS) and/or hospitalisation/emergency room care due to asthma and/or a decrease in peak expiratory flow (PEF) of ≥30% on two consecutive days compared with the run-in period. In these studies, exacerbations were to be treated with a 10-day OCS course; an exacerbation lasting >10 days was considered a new exacerbation on the 11th day. Based on this experience, the later three studies 13-15 did not include falls in PEF in the exacerbation definition, nor this stipulated OCS treatment time, defining a severe exacerbation as the need for OCS for ≥3 days and/or hospitalisation/emergency room care due to asthma worsening. For the purpose of this analysis, we defined a severe exacerbation as the need for OCS (for ≥3 days 10-12) and/or hospitalisation/emergency room care due to asthma worsening; to align with this definition, data from 3 of the included studies 13-15 were reanalysed to exclude PEF fall from the exacerbation definition. 1 SMART Adolescent manuscript: Supplementary materials 07/09/2017 Literature review to identify any additional studies We conducted a review to identify any additional randomised controlled trials (RCTs) evaluating a combination of inhaled corticosteroids (ICS) and a rapid- acting bronchodilator in a single inhaler for both maintenance and as-needed relief of symptoms -

Clenbuterol Elisa Kit Instructions Product #101219 & 101216 Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology CLENBUTEROL ELISA KIT INSTRUCTIONS PRODUCT #101219 & 101216 FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Clenbuterol and/or other metabolites in human urine, blood, oral fluid. DESCRIPTION Neogen Corporation’s Clenbuterol ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug- enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the diluted drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed 3 times to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of Red Stop Solution. -

Application Number
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 203975s000 SUMMARY REVIEW SUMMARY REVIEW OF REGULATORY ACTION Date: November 26, 2013 From: Badrul A. Chowdhury, MD, PhD Director, Division of Pulmonary, Allergy, and Rheumatology Products, CDER, FDA Subject: Division Director Summary Review NDA Number: 20-3975 Applicant Name: GlaxoSmithKline Date of Submission: December 18, 2012 PDUFA Goal Date: December 18, 2013 Proprietary Name: Anoro Ellipta Established Name: Umeclidinium and vilanterol Dosage form: Inhalation Powder (inhaler contains 2 double-foil blister strips, each with 30 blisters containing powder for oral inhalation) Strength: Umeclidinium 62.5 mcg per blister and vilanterol 25 mcg per blister Proposed Indications: Maintenance treatment of airflow obstruction in chronic obstructive pulmonary disease (COPD) Action: Approval 1. Introduction GlaxoSmithKline (GSK) submitted this 505(b)(1) new drug application for use of Anoro Ellipta (umeclidinium 62.5 mcg and vilanterol 25 mcg inhalation powder) for long-term once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD). The proposed dose is one inhalation (umeclidinium 62.5 mcg and vilanterol 25 mcg) once daily. The application is based on clinical efficacy and safety studies. This summary review will provide an overview of the application, with a focus on the clinical efficacy and safety studies. 2. Background There are several drug classes available for the relief of airflow obstruction in patients with COPD. These include short- and long-acting beta-2 adrenergic agonists, short- and long-acting anticholinergics, combination products containing beta-2 adrenergic agonists and anticholinergics, combination of long-acting beta-2 adrenergic agonists and corticosteroids, methylxanthines, and phosphodiesterase-4 (PDE4) inhibitors. -

Asthma Medications
Relievers / Rescue / Bronchodilators Asthma Short-acting Beta Agonists 2 Medications Ipratropium Bromide ProAir ProAir RespiClick Proventil Ventolin Xopenex albuterol sulfate albuterol sulfate dry powder albuterol sulfate albuterol sulfate levalbuterol tartrate 90mcg 90mcg 90mcg 90mcg 45mcg Teva Teva Merck GlaxoSmithKline Sunovion Atrovent* Combivent Respimat* ipratropium bromide ipratropium bromide 20mcg, 17mcg albuterol sulfate 100mcg Boehringer Ingelheim Boehringer Ingelheim Nebulized Albuterol Xopenex Inhalation Solution Xopenex Inhalation Solution Xopenex Inhalation Solution * Ipratropium bromide is not a recommended rescue inhaler albuterol sulfate levalbuterol HCl levalbuterol HCl levalbuterol HCl outside of use in the emergency room or urgent care but may, 2.5mg/3mL 0.31mg/3mL 0.63mg/3mL 1.25mg/3mL on occasion, be prescribed to supplement short-acting Beta 2 generic Sunovion Sunovion Sunovion agonists. Controllers Inhaled Corticosteroids (ICS): Metered-Dose Inhalers (MDI) Aerospan Alvesco Alvesco Asmanex Asmanex Flovent Flovent Flovent flunisolide ciclesonide ciclesonide mometasone furoate mometasone furoate fluticasone propionate fluticasone fluticasone propionate 80mcg 80mcg 160mcg 100mcg 200mcg 44mcg propionate 220mcg Meda Pharmaceuticals Sunovion Sunovion Merck Merck GlaxoSmithKline 110mcg GlaxoSmithKline GlaxoSmithKline Inhaled Corticosteroids (ICS): Dry Powder Inhalers(continued on back) QVAR QVAR beclomethasone beclomethasone dipropionate dipropionate 40mcg 80mcg ArmonAir RespiClick ArmonAir RespiClick ArmonAir RespiClick -

Beta Adrenergic Agents
Beta2 Adrenergic Agents –Long Acting Review 04/10/2008 Copyright © 2007 - 2008 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 5181 Natorp Blvd., Suite 205 Mason, Ohio 45040 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Comments and suggestions may be sent to [email protected]. -

Orciprenaline Sulphate (Alupent): Planned Withdrawal from the UK Market Following a Risk-Benefit Analysis
MHRA PUBLIC ASSESSMENT REPORT Orciprenaline sulphate (Alupent): planned withdrawal from the UK market following a risk-benefit analysis November 2009 Executive summary 2 1. Introduction 3 2. Summary of data 3 2.1 Clinical pharmacology 3 2.2 Efficacy 3 2.3 Safety 4 3. Conclusions 8 4. References 9 5. Glossary 10 1 EXECUTIVE SUMMARY (Please note that this summary is intended to be accessible to all members of the public, including health professionals) Background The Medicines and Healthcare products Regulatory Agency (MHRA) is the government agency responsible for regulating the effectiveness and safety of medicines and medical devices in the UK. We continually review the safety of all medicines in the UK, and inform healthcare professionals and the public of the latest safety updates. In our Public Assessment Reports, we discuss the evidence for a safety issue with a particular drug or drug class, and changes made to the product information for the drug on the basis of this evidence, which will help safeguard public health. This MHRA Public Assessment Report discusses a review of the risks and benefits of a medicine called orciprenaline sulphate. Orciprenaline sulphate is available for oral administration as a syrup used to treat reversible airways obstructiona, which is a symptom of asthmab and chronic obstructive pulmonary diseasec. It acts on specific areas in the body called β- receptors, which relaxes the muscles used for breathing and opens the airways in the lungs. Orciprenaline sulphate was licensed in 1972 and is marketed in the UK under the brand name Alupent Syrup. As with any medicine, the use of orciprenaline sulphate may lead to adverse reactions (side-effects) in some individuals, which are described in the product information, including the patient information leaflet (see the Electronic Medicines Compendium (product information) website).