FUNCTIONAL EXPLORATION and CHARACTERIZATION of the DEAMINASES of COG0402 a Dissertation by DANIEL STEPHEN HITCHCOCK Submitted To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Downloaded 10/5/2021 9:43:05 AM
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Aromatic side-chain flips orchestrate the conformational sampling of functional loops in Cite this: Chem. Sci.,2021,12,9318 † All publication charges for this article human histone deacetylase 8 have been paid for by the Royal Society a a bcd of Chemistry Vaibhav Kumar Shukla, ‡ Lucas Siemons, ‡ Francesco L. Gervasio and D. Flemming Hansen *a Human histone deacetylase 8 (HDAC8) is a key hydrolase in gene regulation and an important drug-target. High-resolution structures of HDAC8 in complex with substrates or inhibitors are available, which have provided insights into the bound state of HDAC8 and its function. Here, using long all-atom unbiased molecular dynamics simulations and Markov state modelling, we show a strong correlation between the conformation of aromatic side chains near the active site and opening and closing of the surrounding functional loops of HDAC8. We also investigated two mutants known to allosterically downregulate the enzymatic activity of HDAC8. Based on experimental data, we hypothesise that I19S-HDAC8 is unable to Received 6th April 2021 Creative Commons Attribution 3.0 Unported Licence. release the product, whereas both product release and substrate binding are impaired in the S39E- Accepted 27th May 2021 HDAC8 mutant. The presented results deliver detailed insights into the functional dynamics of HDAC8 DOI: 10.1039/d1sc01929e and provide a mechanism for the substantial downregulation caused by allosteric mutations, including rsc.li/chemical-science a disease causing one. Introduction II (HDAC-4, -5, -6, -7, -9, and HDAC10), and class III (SIRT1-7) have sequence similarity to yeast Rpd3, Hda1, and Sir2, Acetylation of lysine side chains occurs as a co-translation or respectively, whereas class IV (HDAC11) shares sequence simi- 2 This article is licensed under a post-translational modication of proteins and was rst iden- larity with both class I and II proteins. -

Structural Insights Into Histone Modifying Enzymes
Wayne State University Wayne State University Theses January 2019 Structural Insights Into Histone Modifying Enzymes Shruti Amle Wayne State University, [email protected] Follow this and additional works at: https://digitalcommons.wayne.edu/oa_theses Part of the Biochemistry Commons, and the Molecular Biology Commons Recommended Citation Amle, Shruti, "Structural Insights Into Histone Modifying Enzymes" (2019). Wayne State University Theses. 693. https://digitalcommons.wayne.edu/oa_theses/693 This Open Access Embargo is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Theses by an authorized administrator of DigitalCommons@WayneState. STRUCTURAL INSIGHTS INTO HISTONE MODIFYING ENZYMES by SHRUTI AMLE THESIS Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE 2019 MAJOR: BIOCHEMISTRY AND MOLECULAR BIOLOGY Approved By: _________________________________________ Advisor Date _________________________________________ _________________________________________ _________________________________________ i ACKNOWLEDGEMENTS Writing this thesis has been extremely captivating and gratifying. I take this opportunity to express my deep and sincere acknowledgements to the number of people for extending their generous support and unstinted help during my entire study. Firstly, I would like to express my respectful regards and deep sense of gratitude to my advisor, Dr. Zhe Yang. I am extremely honored to study and work under his guidance. His vision, ideals, timely motivation and immense knowledge had a deep influence on my entire journey of this career. Without his understanding and support, it would not have been possible to complete this research successfully. I also owe my special thanks to my committee members: Dr. -

Purification Andsomeproperties of Cytosine Deaminase from Bakers
Agric. Biol. Chem., 53 (5), 1313-1319, 1989 1313 Purification and SomeProperties of Cytosine Deaminase from Bakers' Yeast Tohoru Katsuragi, Toshihiro Sonoda, Kin'ya Matsumoto, Takuo Sakai and Kenzo Tonomura Laboratory of Fermentation Chemistry, College of Agriculture, University of Osaka Prefecture, Sakai-shi, Osaka 591, Japan Received November 24, 1988 Cytosine deaminase (EC 3.5.4.1) was extracted from commercial compressed bakers' yeast and purified to an almost homogeneous state. The enzyme activity was more than 200U/mg of protein, which was several times higher than reported before. The molecular weight was 41,000 by gel permeation. The pi was at pH4.7. 5-Fluorocytosine, 5-methylcytosine, and creatinine were other substrates for the enzyme.An experiment with inhibitors suggested that the enzyme was an SH- enzyme. The enzyme was unstable to heat, with a half-life of about 0.5hr at 37°C. Characteristics of the enzyme, especially its substrate specificity, were compared with those reported earlier for other cytosine deaminases from bacteria and a mold. Local chemotherapy of cancer with the com- (5MC), a 5-substituted cytosine.4) 5FC, an- bined use of 5-fluorocytosine (5FC) given oral- other 5-substituted cytosine, is deaminated to ly and a cytosine deaminase capsule implant- 5FU in Saccharomyces cerevisiae.5) So, cy- ed locally may be possible.1} However, al- tosine deaminase of bakers' yeast should con- though this approach is successful in animal vert 5FCto 5FU, and could be used in place of experiments,1'2) there are problems when we E. coli cytosine deaminase. Although the yeast use the enzyme from Escherichia coli,3) which enzyme is unstable to heat (at 37.5°C),4) which is thermostable,1'3) and which can deaminate would prevent its use in long-term therapy in 5FC to 5-fluorouracil (5FU).1>3) First, it is the body, it might be stabilized by immobili- difficult to culture the bacteria on a large scale zation or other techniques. -

Molecular Basis of NDT-Mediated Activation of Nucleoside-Based Prodrugs and Application in Suicide Gene Therapy
biomolecules Article Molecular Basis of NDT-Mediated Activation of Nucleoside-Based Prodrugs and Application in Suicide Gene Therapy Javier Acosta 1,† , Elena Pérez 1,†, Pedro A. Sánchez-Murcia 2, Cristina Fillat 3,4 and Jesús Fernández-Lucas 2,5,* 1 Applied Biotechnology Group, European University of Madrid, c/ Tajo s/n, Villaviciosa de Odón, 28670 Madrid, Spain; [email protected] (J.A.); [email protected] (E.P.) 2 Division of Physiological Chemistry, Otto-Loewi Research Center, Medical University of Graz, Neue Stiftingtalstraße 6/III, A-8010 Graz, Austria; [email protected] 3 Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), 08036 Barcelona, Spain; cfi[email protected] 4 Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), 08036 Barcelona, Spain 5 Grupo de Investigación en Ciencias Naturales y Exactas, GICNEX, Universidad de la Costa, CUC, Calle 58 # 55-66 Barranquilla, Colombia * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Herein we report the first proof for the application of type II 20-deoxyribosyltransferase from Lactobacillus delbrueckii (LdNDT) in suicide gene therapy for cancer treatment. To this end, we first confirm the hydrolytic ability of LdNDT over the nucleoside-based prodrugs 20-deoxy-5- fluorouridine (dFUrd), 20-deoxy-2-fluoroadenosine (dFAdo), and 20-deoxy-6-methylpurine riboside (d6MetPRib). Such activity was significantly increased (up to 30-fold) in the presence of an acceptor nucleobase. To shed light on the strong nucleobase dependence for enzymatic activity, different molecular dynamics simulations were carried out. Finally, as a proof of concept, we tested the LdNDT/dFAdo system in human cervical cancer (HeLa) cells. -

35 Disorders of Purine and Pyrimidine Metabolism
35 Disorders of Purine and Pyrimidine Metabolism Georges van den Berghe, M.- Françoise Vincent, Sandrine Marie 35.1 Inborn Errors of Purine Metabolism – 435 35.1.1 Phosphoribosyl Pyrophosphate Synthetase Superactivity – 435 35.1.2 Adenylosuccinase Deficiency – 436 35.1.3 AICA-Ribosiduria – 437 35.1.4 Muscle AMP Deaminase Deficiency – 437 35.1.5 Adenosine Deaminase Deficiency – 438 35.1.6 Adenosine Deaminase Superactivity – 439 35.1.7 Purine Nucleoside Phosphorylase Deficiency – 440 35.1.8 Xanthine Oxidase Deficiency – 440 35.1.9 Hypoxanthine-Guanine Phosphoribosyltransferase Deficiency – 441 35.1.10 Adenine Phosphoribosyltransferase Deficiency – 442 35.1.11 Deoxyguanosine Kinase Deficiency – 442 35.2 Inborn Errors of Pyrimidine Metabolism – 445 35.2.1 UMP Synthase Deficiency (Hereditary Orotic Aciduria) – 445 35.2.2 Dihydropyrimidine Dehydrogenase Deficiency – 445 35.2.3 Dihydropyrimidinase Deficiency – 446 35.2.4 Ureidopropionase Deficiency – 446 35.2.5 Pyrimidine 5’-Nucleotidase Deficiency – 446 35.2.6 Cytosolic 5’-Nucleotidase Superactivity – 447 35.2.7 Thymidine Phosphorylase Deficiency – 447 35.2.8 Thymidine Kinase Deficiency – 447 References – 447 434 Chapter 35 · Disorders of Purine and Pyrimidine Metabolism Purine Metabolism Purine nucleotides are essential cellular constituents 4 The catabolic pathway starts from GMP, IMP and which intervene in energy transfer, metabolic regula- AMP, and produces uric acid, a poorly soluble tion, and synthesis of DNA and RNA. Purine metabo- compound, which tends to crystallize once its lism can be divided into three pathways: plasma concentration surpasses 6.5–7 mg/dl (0.38– 4 The biosynthetic pathway, often termed de novo, 0.47 mmol/l). starts with the formation of phosphoribosyl pyro- 4 The salvage pathway utilizes the purine bases, gua- phosphate (PRPP) and leads to the synthesis of nine, hypoxanthine and adenine, which are pro- inosine monophosphate (IMP). -

Purine Metabolism in Cultured Endothelial Cells
PURINE METABOLISM IN MAN-III Biochemical, Immunological, and Cancer Research Edited by Aurelio Rapado Fundacion Jimenez Diaz Madrid, Spain R.W.E. Watts M.R.C. Clinical Research Centre Harrow, England and Chris H.M.M. De Bruyn Department of Human Genetics University of Nijmegen Faculty of Medicine Nijmegen, The Netherlands PLENUM PRESS · NEW YORK AND LONDON Contents of Part Β I. PURINE METABOLISM PATHWAYS AND REGULATION A. De Novo Synthesis; Precursors and Regulation De Novo Purine Synthesis in Cultured Human Fibroblasts 1 R.B. Gordon, L. Thompson, L.A. Johnson, and B.T. Emmerson Comparative Metabolism of a New Antileishmanial Agent, Allopurinol Riboside, in the Parasite and the Host Cell 7 D. J. Nelson, S.W. LaFon, G.B. Elion, J.J. Marr, and R.L. Berens Purine Metabolism in Rat Skeletal Muscle 13 E. R. Tully and T.G. Sheehan Alterations in Purine Metabolism in Cultured Fibroblasts with HGPRT Deficiency and with PRPPP Synthetase Superactivity 19 E. Zoref-Shani and 0. Sperling Purine Metabolism in Cultured Endothelial Cells 25 S. Nees, A.L. Gerbes, B. Willershausen-Zönnchen, and E. Gerlach Determinants of 5-Phosphoribosyl-l-Pyrophosphate (PRPP) Synthesis in Human Fibroblasts 31 K.0, Raivio, Ch. Lazar, H. Krumholz, and M.A. Becker Xanthine Oxidoreductase Inhibition by NADH as a Regulatory Factor of Purine Metabolism 35 M.M. Jezewska and Z.W. Kaminski vii viii CONTENTS OF PART Β Β. Nucleotide Metabolism Human Placental Adenosine Kinase: Purification and Characterization 41 CM. Andres, T.D. Palella, and I.H. Fox Long-Term Effects of Ribose on Adenine Nucleotide Metabolism in Isoproterenol-Stimulated Hearts . -

Association Between the Gut Microbiota and Blood Pressure in a Population Cohort of 6953 Individuals
Journal of the American Heart Association ORIGINAL RESEARCH Association Between the Gut Microbiota and Blood Pressure in a Population Cohort of 6953 Individuals Joonatan Palmu , MD; Aaro Salosensaari , MSc; Aki S. Havulinna , DSc (Tech); Susan Cheng , MD, MPH; Michael Inouye, PhD; Mohit Jain, MD, PhD; Rodolfo A. Salido , BSc; Karenina Sanders , BSc; Caitriona Brennan, BSc; Gregory C. Humphrey, BSc; Jon G. Sanders , PhD; Erkki Vartiainen , MD, PhD; Tiina Laatikainen , MD, PhD; Pekka Jousilahti, MD, PhD; Veikko Salomaa , MD, PhD; Rob Knight , PhD; Leo Lahti , DSc (Tech); Teemu J. Niiranen , MD, PhD BACKGROUND: Several small-scale animal studies have suggested that gut microbiota and blood pressure (BP) are linked. However, results from human studies remain scarce and conflicting. We wanted to elucidate the multivariable-adjusted as- sociation between gut metagenome and BP in a large, representative, well-phenotyped population sample. We performed a focused analysis to examine the previously reported inverse associations between sodium intake and Lactobacillus abun- dance and between Lactobacillus abundance and BP. METHODS AND RESULTS: We studied a population sample of 6953 Finns aged 25 to 74 years (mean age, 49.2±12.9 years; 54.9% women). The participants underwent a health examination, which included BP measurement, stool collection, and 24-hour urine sampling (N=829). Gut microbiota was analyzed using shallow shotgun metagenome sequencing. In age- and sex-adjusted models, the α (within-sample) and β (between-sample) diversities of taxonomic composition were strongly re- lated to BP indexes (P<0.001 for most). In multivariable-adjusted models, β diversity was only associated with diastolic BP (P=0.032). -

Metabolism of Purines and Pyrimidines in Health and Disease
39th Meeting of the Polish Biochemical Society Gdañsk 16–20 September 2003 SESSION 6 Metabolism of purines and pyrimidines in health and disease Organized by A. C. Sk³adanowski, A. Guranowski 182 Session 6. Metabolism of purines and pyrimidines in health and disease 2003 323 Lecture The role of DNA methylation in cytotoxicity mechanism of adenosine analogues in treatment of leukemia Krystyna Fabianowska-Majewska Zak³ad Chemii Medycznej IFiB, Uniwersytet Medyczny, ul. Mazowiecka 6/8, 92 215 £ódŸ Changes in DNA methylation have been recognized tory effects of cladribine and fludarabine on DNA as one of the most common molecular alterations in hu- methylation, after 48 hr growth of K562 cells with the man neoplastic diseases and hypermethylation of drugs, are non-random and affect mainly CpG rich is- gene-promoter regions is one of the most frequent lands or CCGG sequences but do not affect sepa- mechanisms of the loss of gene functions. For this rea- rately-located CpG sequences. The analysis showed son, DNA methylation may be a tool for detection of that cladribine (0.1 mM) reduced the methylated early cell transformations as well as predisposition to cytosines in CpG islands and CCGG sequences to a sim- metastasis process. Moreover, DNA methylation seems ilar degree. The inhibition of cytosine methylation by to be a promissing target for new preventive and thera- fludarabine (3 mM) was observed mainly in CCGG se- peutic strategies. quences, sensitive to HpaII, but the decline in the meth- Our studies on DNA methylation and cytotoxicity ylated cytosine, located in CpG island was 2-fold lower mechanism of antileukemic drugs, cladribine and than that with cladribine. -

Structural Properties of the Nickel Ions in Urease: Novel Insights Into the Catalytic and Inhibition Mechanisms
Coordination Chemistry Reviews 190–192 (1999) 331–355 www.elsevier.com/locate/ccr Structural properties of the nickel ions in urease: novel insights into the catalytic and inhibition mechanisms Stefano Ciurli a,*, Stefano Benini b, Wojciech R. Rypniewski b, Keith S. Wilson c, Silvia Miletti a, Stefano Mangani d a Institute of Agricultural Chemistry, Uni6ersity of Bologna, Viale Berti Pichat 10, I-40127 Bologna, Italy b European Molecular Biology Laboratory, c/o DESY, Notkestraße 85, D-22603 Hamburg, Germany c Department of Chemistry, Uni6ersity of York, Heslington, York YO15DD, UK d Department of Chemistry, Uni6ersity of Siena, Pian dei Mantellini 44, I-53100 Siena, Italy Accepted 13 March 1999 Contents Abstract.................................................... 331 1. Biological background ......................................... 332 2. Spectroscopic investigations of the urease active site structure .................. 333 3. Crystallographic studies of the native enzyme ............................ 334 4. Crystallographic studies of urease mutants.............................. 341 5. Crystallographic studies of urease–inhibitor complexes ...................... 345 6. Crystallographic study of a transition state analogue bound to urease.............. 348 7. A novel proposal for the urease mechanism ............................. 350 References .................................................. 353 Abstract This work provides a comprehensive critical summary of urease spectroscopy, crystallogra- phy, inhibitor binding, and site-directed -

Supplementary Table S1. Table 1. List of Bacterial Strains Used in This Study Suppl
Supplementary Material Supplementary Tables: Supplementary Table S1. Table 1. List of bacterial strains used in this study Supplementary Table S2. List of plasmids used in this study Supplementary Table 3. List of primers used for mutagenesis of P. intermedia Supplementary Table 4. List of primers used for qRT-PCR analysis in P. intermedia Supplementary Table 5. List of the most highly upregulated genes in P. intermedia OxyR mutant Supplementary Table 6. List of the most highly downregulated genes in P. intermedia OxyR mutant Supplementary Table 7. List of the most highly upregulated genes in P. intermedia grown in iron-deplete conditions Supplementary Table 8. List of the most highly downregulated genes in P. intermedia grown in iron-deplete conditions Supplementary Figures: Supplementary Figure 1. Comparison of the genomic loci encoding OxyR in Prevotella species. Supplementary Figure 2. Distribution of SOD and glutathione peroxidase genes within the genus Prevotella. Supplementary Table S1. Bacterial strains Strain Description Source or reference P. intermedia V3147 Wild type OMA14 isolated from the (1) periodontal pocket of a Japanese patient with periodontitis V3203 OMA14 PIOMA14_I_0073(oxyR)::ermF This study E. coli XL-1 Blue Host strain for cloning Stratagene S17-1 RP-4-2-Tc::Mu aph::Tn7 recA, Smr (2) 1 Supplementary Table S2. Plasmids Plasmid Relevant property Source or reference pUC118 Takara pBSSK pNDR-Dual Clonetech pTCB Apr Tcr, E. coli-Bacteroides shuttle vector (3) plasmid pKD954 Contains the Porpyromonas gulae catalase (4) -
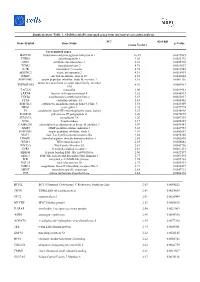
Supplementary Table 1. All Differentially Expressed Genes from Microarray Screening Analysis
Supplementary Table 1. All differentially expressed genes from microarray screening analysis. FCa (Gal-KD Gene Symbol Gene Name p-Value versus Vector) Up-regulated genes HAPLN1 hyaluronan and proteoglycan link protein 1 10.49 0.0027085 THBS1 thrombospondin 1 9.20 0.0022192 ODC1 ornithine decarboxylase 1 6.38 0.0055776 TGM2 transglutaminase 2 4.76 0.0015627 IL7R interleukin 7 receptor 4.75 0.0017245 SERINC2 serine incorporator 2 4.51 0.0014919 ITM2C integral membrane protein 2C 4.32 0.0044644 SERPINB7 serpin peptidase inhibitor, clade B, member 7 4.18 0.0081136 tumor necrosis factor receptor superfamily, member TNFRSF10D 4.01 0.0085561 10d TAGLN transgelin 3.90 0.0099963 LRRN4 leucine rich repeat neuronal 4 3.82 0.0046513 TGFB2 transforming growth factor beta 2 3.51 0.0035017 CPA4 carboxypeptidase A4 3.43 0.0008452 EPB41L3 erythrocyte membrane protein band 4.1-like 3 3.34 0.0025309 NRG1 neuregulin 1 3.28 0.0079724 F3 coagulation factor III (thromboplastin, tissue factor) 3.27 0.0038968 POLR3G polymerase III polypeptide G 3.26 0.0070675 SEMA7A semaphorin 7A 3.20 0.0087335 NT5E 5-nucleotidase 3.17 0.0036353 CAMK2N1 calmodulin-dependent protein kinase II inhibitor 1 3.07 0.0090141 TIMP3 TIMP metallopeptidase inhibitor 3 3.03 0.0047953 SERPINE1 serpin peptidase inhibitor, clade E 2.97 0.0053652 MALL mal, T-cell differentiation protein-like 2.88 0.0078205 DDAH1 dimethylarginine dimethylaminohydrolase 1 2.86 0.0002895 WDR3 WD repeat domain 3 2.85 0.0058842 WNT5A Wnt Family Member 5A 2.81 0.0043796 GPR1 G protein-coupled receptor 1 2.81 0.0021313 -

(12) United States Patent (10) Patent No.: US 9.422,609 B2 Teichberg (45) Date of Patent: Aug
USOO9422609B2 (12) United States Patent (10) Patent No.: US 9.422,609 B2 Teichberg (45) Date of Patent: Aug. 23, 2016 (54) METHODS, COMPOSITIONS AND DEVICES (58) Field of Classification Search FOR MANTAINING CHEMICAL BALANCE CPC ........................ C02F 1/725; C12Y 305/01005 OF CHLORINATED WATER USPC ........................... 210/754; 435/195, 227 231 See application file for complete search history. (75) Inventor: Vivian I. Teichberg, Savyon (IL) (56) References Cited (73) Assignees: Mia Levite, Savyon (IL); Yaar Teichberg, Savyon (IL); Nof Lyle U.S. PATENT DOCUMENTS Teichberg, Savyon (IL) 4,793,935 A * 12/1988 Stillman ............... CO2F 1.5236 21Of727 (*) Notice: Subject to any disclaimer, the term of this 6,673,582 B2 * 1/2004 McTavish ..................... 435/122 patent is extended or adjusted under 35 U.S.C. 154(b) by 1044 days. (Continued) (21) Appl. No.: 12/225.18O FOREIGN PATENT DOCUMENTS y x- - - 9 AU 41971 5, 1979 (22) PCT Filed: Mar. 14, 2007 GB 2025919 1, 1980 (86). PCT No.: PCT/L2007/OOO336 (Continued) S 371 (c)(1) OTHER PUBLICATIONS (2), (4) Date: Sep. 16, 2008 Examiner's Report Dated Oct. 6, 2010 From the Australian Govern (87) PCT Pub. No.: WO2007/107.981 ment, IP Australia Re. Application No. 2007228391. (Continued) PCT Pub. Date: Sep. 27, 2007 (65) Prior Publication Data Primary Examiner — Peter Keyworth (74) Attorney, Agent, or Firm — Browdy and Neimark, US 201OfO270228A1 Oct. 28, 2010 PLLC Related U.S. Application Data (57) ABSTRACT (60) Provisional application No. 60/783,028, filed on Mar. A composition-of-matter for use in water treatment, com 17, 2006.