Plastome Comparative Genomics in Maples Resolves the Infrageneric Backbone Relationships
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -
Seeds and Plants Imported
Issued November 9,1915. U. S. DEPARTMENT OF AGRICULTURE. BUREAU OF PLANT INDUSTRY. WILLIAM A. TAYLOR, Chief of Bureau. INVENTORY SEEDS AND PLANTS IMPORTED OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM APRIL 1 " " TO JUNE 30,1913. " , r (No. 35; Nos. 35136 TO 3566^..,--"•-****"*"' WASHINGTON: GOVERNMENT PRINTING OFFICE. 1915. Issued November 9,1915. U. S. DEPARTMENT OF AGRICULTURE. BUREAU OF PLANT INDUSTRY. WILLIAM A. TAYLOR, Chief of Bureau. INVENTORY SEEDS AND PLANTS IMPORTED BY THE OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM APRIL 1 TO JUNE 30,1913. (No. 35; Nos. 35136 TO 35666.) WASHINGTON: GOVERNMENT PRINTING OFFICE. 1915. BUREAU OF PLANT INDUSTRY. Chief of Bureau, WILLIAM A. TAYLOR. Assistant Chief of Bureau, KARL F. KELLERMAN. Officer in Charge of Publications, J. E. ROCKWELL. Chief Clerk, JAMES E. JONES. FOREIGN SEED AND PLANT INTRODUCTION. SCIENTIFIC STAFF. David Fairchild, Agricultural Explorer in Charge. P. H. Dorsett, Plant Introducer, in Charge of Plant Introduction Field Stations. Peter Bisset, Plant Introducer, in Charge of Foreign Plant Distribution. Frank N. Meyer and Wilson Popenoe, Agricultural Explorers. H. C. Skeels, S. C. Stuntz, and R. A. Young, Botanical Assistants. Allen M. Groves, Nathan Menderson, and Glen P. Van Eseltine, Assistants. Robert L. Beagles, Superintendent, Plant Introduction Field Station, Chico, Cal. Edward Simmonds, Superintendent, Subtropical Plant Introduction Field Station, Miami, Fla. John M. Rankin, Superintendent, Yarrow Plant Introduction Field Station, Rockville, Md. E. R. Johnston, Assistant in Charge, Plant Introduction Field Station, Brooksville, Fla. Edward Goucher and H. Klopfer, Plant Propagators. Collaborators: Aaron Aaronsohn, Director, Jewish Agricultural Experimental Station, Haifa, Palestine; Thomas W. -
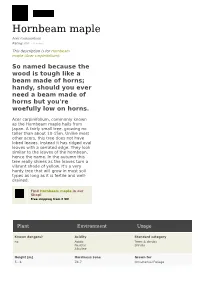
Hornbeam Maple Acer Carpinifolium Rating: 0.0 ( 0 Votes)
Hornbeam maple Acer carpinifolium Rating: 0.0 ( 0 votes) This description is for Hornbeam maple (Acer carpinifolium): So named because the wood is tough like a beam made of horns; handy, should you ever need a beam made of horns but you're woefully low on horns. Acer carpinifolium, commonly known as the Hornbeam maple hails from Japan. A fairly small tree, growing no taller than about 10-15m. Unlike most other acers, this tree does not have lobed leaves. Instead it has ridged oval leaves with a serrated edge. They look similar to the leaves of the hornbean, hence the name. In the autumn this tree really shines as the leaves turn a vibrant shade of yellow. It's a very hardy tree that will grow in most soil types as long as it is fertile and well- drained. Find Hornbeam maple in our Shop! Free shipping from € 50! Plant Environment Usage Known dangers? Acidity Standard category no Acidic Trees & shrubs Neutral Shrubs Alkaline Height [m] Hardiness zone Grown for 5 - 6 Z4-7 Ornamental Foliage Plant Environment Usage Spread [m] Heat zone Creative category 4 H7-1 Kid Approved For Beginners Show-offs Dominant flower colour Winter temperatures [°C] Garden type Green -34 - -12 Woodland Park City Flower Fragrance Heat days Garden spaces No, neutral please 0 - 90 Specimen Flowering seasons Moisture Gardening expertise Early spring well-drained but frequently watered beginner Mid spring Foliage in spring Soil type Time to reach full size Green sandy up to 20 years Clay chalky loams Foliage in summer Sun requirements Green Full sun Partial shade Foliage in Autumn Exposure Red shades Sheltered Propagation methods grafting seed budding . -

Acer Glabrum Var. Douglasii Snowberry
NATIVE PLANTS/SHRUBS: Saskatoon Serviceberry – Amielanchier alnifolia Oceanspray – Holodiscus discolor Blue Elderberry - Sambucus cerulea Erect, loosely branched shrub up to 15’ tall. Found Usually a shrub or small tree from 10’ to 20’ or Vine Maple - Acer circinatum Large shrubs or small trees up to 40’ tall and 6” in diameter. Occurs on moist, well-drained on well drained to dry sites in the sun and shade more tall. Found on moist, well-drained sites in An erect shrub, or more commonly a helter- sites in the sun or partial shade, from southern from south central California northward to British the sun; from British Columbia south to California, skelter arrangement of crooked branches; Alaska to northwestern California, and east- Columbia, eastward to Idaho. east through Idaho, Utah, and Nevada. up to 20’ tall, or less commonly a small tree ward throughout the Rocky Mountains. Elevational range: sea level to 9,000 feet. 30’ to 40’ in height. Common understory Dull Oregon Grape – Mahonia (Berberis) nervosa species in the West side forests of the Red Elderberry – Sambucus racemosa Pacific Northwest also pioneer species on Oregon Grape – Low, evergreen shrub with pinnately Berberis aquifolium (Tall Oregon Grape) A shrub or small tree from 8’ to 20’ tall. Occurs on cutover and burned-over lands. Found compound leaves; seldom over 30” high. moist, well-drained sites in the sun; most Erect evergreen shrub 3’ to 10’ tall, with dark on moist sites in the sun or shade from Grows on moist, well-drained sites in the sun common on the West Side of the Cascade green glossy leaves. -

Experience 2,000 Follow the Path from the Roundabout Towards East, Past the Small Lake and You Will Be at the Main Entrance
The Arboretum in Hørsholm Why an Arboretum? Te Arboretum is a living collection of more than 8,500 trees Less than a hundred species of trees, shrubs and woody climbers and shrubs. Te objective of the collection is to include and are native to Denmark. Te main reason for this is the fact that observe woody plant species that can survive and grow under most of Northern Europe during each of the last 7 major glacia- Danish conditions. tions was covered by ice, and the unglaciated landscapes north of the Alps were covered by arctic and sub-arctic tundras and steppes. All plants in the Arboretum have detailed documentation of age, Te rich fora in e.g. North America and Eastern Asia could escape GPS location, species identity, and the geographic origin of where such cold and dry climates. seeds were collected. Te information is stored in a database and can be accessed from the Arboretum webpage. In spite of our poor native dendrofora, a rich variety of exotic species and provenances are able to grow in the Danish climate. Te collection is managed by the Department of Geosciences At present the collections of the Arboretum include approximately Cornus kousa and Natural Resource Management, University of Copenhagen. 2,000 species, subspecies and cultivars in an area of 25 ha. Te Arboretum is a continuation of the Forest Botanical Garden in reveals if the seed was collected from a natural population (W), Charlottenlund from 1838. Te establishment of the Arboretum in from a plant that originates from a natural population (Z) Hrsholm was initiated in 1936, to support teaching and research or is a cultivated variety (G). -

Native Or Suitable Plants City of Mccall
Native or Suitable Plants City of McCall The following list of plants is presented to assist the developer, business owner, or homeowner in selecting plants for landscaping. The list is by no means complete, but is a recommended selection of plants which are either native or have been successfully introduced to our area. Successful landscaping, however, requires much more than just the selection of plants. Unless you have some experience, it is suggested than you employ the services of a trained or otherwise experienced landscaper, arborist, or forester. For best results it is recommended that careful consideration be made in purchasing the plants from the local nurseries (i.e. Cascade, McCall, and New Meadows). Plants brought in from the Treasure Valley may not survive our local weather conditions, microsites, and higher elevations. Timing can also be a serious consideration as the plants may have already broken dormancy and can be damaged by our late frosts. Appendix B SELECTED IDAHO NATIVE PLANTS SUITABLE FOR VALLEY COUNTY GROWING CONDITIONS Trees & Shrubs Acer circinatum (Vine Maple). Shrub or small tree 15-20' tall, Pacific Northwest native. Bright scarlet-orange fall foliage. Excellent ornamental. Alnus incana (Mountain Alder). A large shrub, useful for mid to high elevation riparian plantings. Good plant for stream bank shelter and stabilization. Nitrogen fixing root system. Alnus sinuata (Sitka Alder). A shrub, 6-1 5' tall. Grows well on moist slopes or stream banks. Excellent shrub for erosion control and riparian restoration. Nitrogen fixing root system. Amelanchier alnifolia (Serviceberry). One of the earlier shrubs to blossom out in the spring. -

Acer Platanoides in Europe: Distribution, Habitat, Usage and Threats
Acer platanoides Acer platanoides in Europe: distribution, habitat, usage and threats G. Caudullo, D. de Rigo Acer platanoides L., commonly known as Norway maple, is a large tree that is widespread in central Europe and reaching eastwards the Ural Mountains. It is a fast-growing species, able to grow in a wide range of soils and habitat conditions. In natural stands it occurs in fresh and humid sites in temperate mixed forests, both with conifers and broadleaves. It is a secondary species, growing in small groups or individually. It has been planted intensively as an ornamental and shade tree, appreciated for its colourful foliage and large, spreading crown, in combination with its tolerance of urban conditions. Its wood is also valued for its attractive flaming figures and is used for music instruments, furniture, marquetry and turned objects. This maple is generally free of serious diseases, except in urban areas, where it is more vulnerable to pathogens. In North America it has been widely planted and is now naturalised, becoming an invasive species. The Norway maple (Acer platanoides L.) is a large and tall- domed tree, sometimes very broad, growing to 25-30 m tall and Frequency 60-80 cm in diameter, although exceptionally over 150 cm. The < 25% 25% - 50% stem is straight, short with perpendicular shoots and the crown 50% - 75% is dense with foliage. The leaves are opposite, simple, 10-15 cm > 75% Chorology long, very variable in dimension depending on the age and the Native vigour of the tree. They have five lobes with long and acuminate Introduced teeth and smooth margins. -

Arboretum News Armstrong News & Featured Publications
Georgia Southern University Digital Commons@Georgia Southern Arboretum News Armstrong News & Featured Publications Spring 2019 Arboretum News Georgia Southern University- Armstrong Campus Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/armstrong-arbor-news Part of the Education Commons This article is brought to you for free and open access by the Armstrong News & Featured Publications at Digital Commons@Georgia Southern. It has been accepted for inclusion in Arboretum News by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. Arboretum News Issue 9 | Spring 2019 A Newsletter of the Georgia Southern University Armstrong Campus Arboretum From the Editor: Arboretum News, published by the Grounds Operations Department ’d like to introduce you to the Armstrong Arboretum of the new of Georgia Southern University- IGeorgia Southern University-Armstrong Campus. Designated Armstrong Campus, is distributed as an on-campus arboretum in 2001 by former Armstrong to faculty, staff, students and Atlantic State University president Dr. Thomas Jones, the friends of the Armstrong Arboretum. The Arboretum university recognized the rich diversity of plant life on campus. encompasses Armstrong’s 268- The Arboretum continues to add to that diversity and strives to acre campus and displays a wide function as a repository for the preservation and the conservation variety of shrubs and other woody of plants from all over the world. We also hope to inspire students, plants. Developed areas of campus faculty, staff and visitors to appreciate the incredible diversity contain native and introduced species of trees and shrubs. Most that plants have to offer. -

Amur Maple Acer Ginnala Maxim., Syn Acer Tataricum Ssp
MN NWAC Risk Common Name Latin Name Assessment Worksheet (04-2011) Amur maple Acer ginnala Maxim., syn Acer tataricum ssp. ginnala Reviewer Affiliation/Organization Date (mm/dd/yyyy) Laura Van Riper, MN Department of Natural Resources, 09/17/2015 Tim Power MN Nursery and Landscape Association Box Question Answer Outcome 1 Is the plant species or genotype non-native? Yes. Amur maple is native to Asia. Go to Box 3 3 Is the plant species, or a related species, Yes. Go to Box 6 documented as being a problem elsewhere? Regulated as a Restricted Invasive Species In Wisconsin (all cultivars exempt) (http://dnr.wi.gov/topic/Invasives/fact/AmurMaple.html). Ranked as moderately invasive in New York (http://www.nyis.info/user_uploads/4a6d0_1db2a_Acer.g innala.NYS.pdf). Listed on Illinois Departments of Natural Resources Exotic Species webpages (http://dnr.state.il.us/education/exoticspecies/amurmaple. htm). NatureServe I rank of Medium/Insignificant (http://explorer.natureserve.org/servlet/NatureServe?sear chName=Acer+ginnala). Listed as potentially invasive, but not banned in Connecticut (http://plants.usda.gov/java/noxious?rptType=State&stat efips=09, http://cipwg.uconn.edu/invasive_plant_list/). 6 Does the plant species have the capacity to Yes. Go to Box 7 establish and survive in Minnesota? 1 Box Question Answer Outcome A. Is the plant, or a close relative, currently Yes. Go to Box 7 established in Minnesota? Amur maple has been widely planted in Minnesota. EDDMaps reports Amur maple as present in 42 counties in Minnesota, especially in the northeastern part of the state (http://eddmaps.org/distribution/uscounty.cfm?sub=3965 ). -
Environmental Factors Controlling the Distribution of Forest Plants with Special Reference to Floral Mixture in the Boreo-Nemora
Environmental Factors Controlling the Distribution of Forest Plants with Special Reference to Floral Mixture in the Title Boreo-Nemoral Ecotone, Hokkaido Island Author(s) Uemura, Shigeru Environmental science, Hokkaido University : journal of the Graduate School of Environmental Science, Hokkaido Citation University, Sapporo, 15(2), 1-54 Issue Date 1993-03-25 Doc URL http://hdl.handle.net/2115/37276 Type bulletin (article) File Information 15(2)_1-54.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP 1 Environ,Sci.,I'Iokl{aidoUniversity 15(2) 1-54 Dec,1992 Environmental Factors Controlling the Distribution of Forest PlaRts with Special Reference to Floral Mixture in the Boreo-Nemoral EcotoRe, Hokkaido Island Shigeru Uemura Department of Biosystem Management, Division of Environmental Conservation, Graduate School of Environmental Science, Hokkaiclo University, Sapporo 060, Japan Abstract Effects of climatic factors on the plant distribution were examined by means of direct gradient analysis, and the relationship of forest flora with Iife form and phytogeographical distribution was exaniined. Subsequently, leaf phenology of forest plants were analyzed to evaluate the adaptive signifi- cance in relation to the environments in forest understory. In the boreo-nemoral forest ecotone, Kokkaido Island, northern Japan, co-occurrence of northern and southern plants in a certain forest site is more notable in the understory than in the crown, and this dates back to the late--Quaternary period, where the decrease in temperature associated with the glacial period forced the unclerstory plants to adapt their life forms or leaf habits to snowcover and to light conditions of the interior forests, I<ey words: Direct gradient analysis; Floral mixture; Leaf phenology; Mixed forest; Phytogoegraphy; Snowcover; Understory Intoroduetion In the upper-middle latitudes of Europe, eastern Asia and eastern North America, the boreal coniferous forest formation confronts to the temperate hardwood forest formation. -

2. ACER Linnaeus, Sp. Pl. 2: 1054. 1753. 枫属 Feng Shu Trees Or Shrubs
Fl. China 11: 516–553. 2008. 2. ACER Linnaeus, Sp. Pl. 2: 1054. 1753. 枫属 feng shu Trees or shrubs. Leaves mostly simple and palmately lobed or at least palmately veined, in a few species pinnately veined and entire or toothed, or pinnately or palmately 3–5-foliolate. Inflorescence corymbiform or umbelliform, sometimes racemose or large paniculate. Sepals (4 or)5, rarely 6. Petals (4 or)5, rarely 6, seldom absent. Stamens (4 or 5 or)8(or 10 or 12); filaments distinct. Carpels 2; ovules (1 or)2 per locule. Fruit a winged schizocarp, commonly a double samara, usually 1-seeded; embryo oily or starchy, radicle elongate, cotyledons 2, green, flat or plicate; endosperm absent. 2n = 26. About 129 species: widespread in both temperate and tropical regions of N Africa, Asia, Europe, and Central and North America; 99 species (61 endemic, three introduced) in China. Acer lanceolatum Molliard (Bull. Soc. Bot. France 50: 134. 1903), described from Guangxi, is an uncertain species and is therefore not accepted here. The type specimen, in Berlin (B), has been destroyed. Up to now, no additional specimens have been found that could help clarify the application of this name. Worldwide, Japanese maples are famous for their autumn color, and there are over 400 cultivars. Also, many Chinese maple trees have beautiful autumn colors and have been cultivated widely in Chinese gardens, such as Acer buergerianum, A. davidii, A. duplicatoserratum, A. griseum, A. pictum, A. tataricum subsp. ginnala, A. triflorum, A. truncatum, and A. wilsonii. In winter, the snake-bark maples (A. davidii and its relatives) and paper-bark maple (A. -

Acer Buergerianum Plants, Adequately Moist in Summer but Well Drained in Winter Is "Trident Maple" a Pretty Small Tree Whose Grace Is Enhanced by the Key to Success
Acer buergerianum plants, adequately moist in summer but well drained in winter is "Trident Maple" A pretty small tree whose grace is enhanced by the key to success. 3m. the small three-lobed leaves. Particularly good autumn colour begins scarlet turning orange-yellow. A good hardy Maple Acer x conspicuum 'Silver Cardinal' tolerant of many less favoured sites. 4m. This Snakebark has the most incredible pink and cream variegated foliage, highlighted by the red petioles and young stems. It Acer circinatum 'Monroe' occurred as a chance seedling of A. pensylvanicum and received A plant I've lusted after for years! Shrubby habit, with deeply an Award of Merit in 1985. Our stock is directly derived from incised light green leaves (even more so than A. japonicum the original seedling in the Windsor Great Park. Unless your soil 'Aconitifolium'). Predominantly yellow autumn colours may is very good, it is safest in dappled shade. 3m. develop some orange. Worthy of a special site. 3m. Acer x conspicuum 'Silver Vein' Acer circinatum 'Pacific Fire' A hybrid between A. davidii George Forrest and A. Imagine the coral bark colour of Acer palmatum 'Sangokaku' pensylvanicum Erythrocladum found at Hilliers about 1960. It is combined with the larger leaves and more tolerant growth arguably the best of the basic snakebarks for garden suitability requirements of this species, and the result is a plant with and good colour with its rich purple and white striped winter awesome potential. Fantastic autumn colour too. bark, becoming green with maturity. 5m. Acer circinatum 'Sunglow' Acer davidii This has been on my "wanted" list ever since I first saw it Delightful small tree noted for dazzling autumn colour and photographed! Apricot coloured young growth matures to attractive white striped purple bark in winter.