Association of Retinol Binding Protein 4 and Transthyretin with Triglyceride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Creating Curriculum of English for Conservative Tourism for Junior Guides to Promote Tourist Attractions in Thailand
English Language Teaching; Vol. 11, No. 3; 2018 ISSN 1916-4742 E-ISSN 1916-4750 Published by Canadian Center of Science and Education Creating Curriculum of English for Conservative Tourism for Junior Guides to Promote Tourist Attractions in Thailand Onsiri Wimontham1 1 English Education Curriculum, Nakhon Ratchasima Rajabhat University, Thailand Correspondence: Onsiri Wimontham, English Education Curriculum, Nakhon Ratchasima Rajabhat University, Thailand. E-mail: [email protected] Received: January 1, 2018 Accepted: February 13, 2018 Online Published: February 15, 2018 doi: 10.5539/elt.v11n3p67 URL: http://doi.org/10.5539/elt.v11n3p67 Abstract This research was supported the research fund of 2017 by Office of the Higher Education Commission of Thailand. The objectives of this research are listed below. 1). To form the model of teaching and learning English for local development by English curriculum (B. Ed.) students’ participation in training on out-of-classroom learning management, which focuses on the students’ English skills improvement along with developing the sense of love of their home towns. 2). To create curriculum of English training for conservative tourism for junior guides in Sung Noen District, Nakhon Ratchasima Province. 3). To promote conservative tourist attractions in Sung Noen District, Nakhon Ratchasima Province among foreign tourists, and to boost the local economy so that young generations can earn income and rely on themselves in the future. An interesting result from the research was more income gained from tourism in Sung Noen District, Nakhon Ratchasima Province between April 2016 and June in the same year. The junior guides’ ability to communicate and provide information about tourism in English was evaluated. -

Page 1 PROGRAM (Finalized Version) DAY 1 (July 7Th, 2016
PROGRAM (Finalized Version) DAY 1 (July 7th, 2016) DAY 2 (July 8th, 2016) Registration 8: Registration 9:00-10:30 Sessions A1, B1 9:00-10:15 Sessions A2, B2, C2 9:00-10:15 Session C1 (Days 1 10:30-10:50 Opening Address* & Tea reception 10:15-10:35 Tea break 10:50-11:50 Sessions D1, E1, F1, Poster 1 10:35-11:50 Sessions D2, E2, F2 - 3 2) 11:50-13:00 Lunch (Restaurant on 2/F) 11:50-13:00 Lunch (Restaurant on 2/F) 0 - 16: 13:00-15:15 Sessions G1, H1, J1 13:00-15:15 Sessions G2, H2, J2 00 15:15-15:35 Tea break 15:15-15:35 Tea break 15:35-17:45 Sessions K1, L1, M1 15:35-17:15 Sessions K2, L2 * Opening Address: Associate Professor Dr. Tanida Phatisena, Director of Research and Development Institute, Nakhon Ratchasima Rajabhat University, Thailand (venue: Kujyaku, 2/F). Session A1: INTERNATIONAL TRADE & FINANCE (venue: Kaede, 2/F) Chair: Grzegorz Mazur (Poznan University of Economics and Business) The Impact of NAFTA on the Trade with Non-member Countries: the Case of Canadian Chinese Trade (s16-060) *Best Paper Award* Speaker: Ying Kong (York University, Canada & Tsinghua University, China) The Impact of Financial and Trade Integration on Business Cycles in Emerging Markets (s16-093) Speaker: Lathaporn Ratanavararak (Chulalongkorn University) The Process of Banking Expansion in Asian Developing Countries: The Case of Nepal in Comparison with Japan (s16-114) Speaker: Shinichiro Maeda (Meijo University) Effects of Foreign Direct Investment on GDP Growth of Myanmar: Analysis for the Year of 1989-2014 (s16-170) Speaker: Ei Ei Phyu (Thammasat University) -

Chaiyaphum.Pdf
Information by: TAT Nakhon Ratchasima Tourist Information Division (Tel. 0 2250 5500 ext. 2141-5) Designed & Printed by: Promotional Material Production Division, Marketing Services Department. The contents of this publication are subject to change without notice. Chaiyaphum 2009 Copyright. No commercial reprinting of this material allowed. January 2009 Free Copy Dok Krachiao (Siam Tulip) 08.00-20.00 hrs. Everyday Tourist information by fax available 24 hrs. Website: www.tourismthailand.org E-mail: [email protected] 43 Thai Silk Products of Ban Khwao Thai silk, Chaiyaphum Contents Transportation 5 Amphoe Thep Sathit 27 Attractions 7 Events and Festivals 30 Amphoe Mueang Chaiyaphum 7 Local Products and Souvenirs 31 Amphoe Nong Bua Daeng 16 Facilities in Chaiyaphum 34 Amphoe Ban Khwao 17 Accommodation 34 Amphoe Nong Bua Rawe 17 Restaurants 37 Amphoe Phakdi Chumphon 19 Interesting Activities 41 Amphoe Khon Sawan 20 Useful Calls 41 Amphoe Phu Khiao 21 Amphoe Khon San 22 52-08-068 E_002-003 new29-10_Y.indd 2-3 29/10/2009 18:29 52-08-068 E_004-043 new25_J.indd 43 25/9/2009 23:07 Thai silk, Chaiyaphum Contents Transportation 5 Amphoe Thep Sathit 27 Attractions 7 Events and Festivals 30 Amphoe Mueang Chaiyaphum 7 Local Products and Souvenirs 31 Amphoe Nong Bua Daeng 16 Facilities in Chaiyaphum 34 Amphoe Ban Khwao 17 Accommodation 34 Amphoe Nong Bua Rawe 17 Restaurants 37 Amphoe Phakdi Chumphon 19 Interesting Activities 41 Amphoe Khon Sawan 20 Useful Calls 41 Amphoe Phu Khiao 21 Amphoe Khon San 22 4 5 Chaiyaphum is a province located at the ridge of the Isan plateau in the connecting area between the Central Region and the North. -

Factors Associated with Quality of Life in the Elderly People with Ability in Sung Noen District, Nakhon Ratchasima Province
Review of Integrative Business and Economics Research, Vol. 6, NRRU special issue 238 Factors Associated with Quality of Life in the Elderly People with Ability in Sung Noen District, Nakhon Ratchasima Province Tanida Phatisena Varaporn Chatpahol Faculty of Public health, Nakhon Ratchasima Rajabhat University, Nakhon Ratchasima,Thailand ABSTRACT This objectives of this cross-sectional analytical research were to study the level of the quality of life and factors associated with quality of life in the elderly people with ability in Sung Noen distric, Nakhon Ratchasima province. The sample group consisted of 334 elderly people which selected by using multistage random sampling. Data collection was done by interview forms based upon the quality of life indicators by the WHO (WHOQOL_BREF_THAI) include 20 items within 4 components. The data was analysed by using percentage, mean, standard deviation and correlation tests: chi-square. The findings showed that overall the quality of life in the elderly people with ability was at a good level. Three components of physical domain, social relationships and the environment were rated at good level. A component of psychological domain was rated at a fair level. There was associations at 95% level of significance between the quality of life and health problems. Recommendation from this study was to the related organization should develop the quality of life of the elderly by promoting mental health and caring their own health care. Keywords : Quality of Life, Elderly, Ability 1. INTRODUCTION Thailand is currently facing an aging society resulting from change in population structure with the decrease in birth and death rate. This phenomena stem from the social and economic development in the previous decades bringing high technology in medicine and public health service, thus, expending the age of people. -

SMILE of THAILAND 14D / 13N (Guaranteed Weekly Departure with Min
1 | P a g e SMILE OF THAILAND 14D / 13N (guaranteed weekly departure with min. 2 travellers – current travel itinerary in 2012) (tour begins in Bangkok – tour ends in Bangkok) 2 | P a g e ITINERARY OUTLINE DAY 01: BANGKOK DAY 02: BANGKOK / NAKHON RATCHASIMA (KHORAT) / PHANOM RUNG DAY 03: NAKHON RATCHASIMA / PHIMAI / PHITSANULOK DAY 04: PHITSANULOK / LAMPANG / LAMPHUN / CHIANG MAI DAY 05: CHIANG MAI DAY 06: CHIANG MAI / PAI / MAE HONG SON DAY 07: MAE HONG SON / KHUN YUAM / MAE LA NOI / MAE SOT DAY 08: MAE SOT / UMPHANG DAY 09: UMPHANG / MAE SOT / SUKHOTHAI DAY 10: SUKHOTHAI / N. SAWAN / SUPHANBURI / KANCHANABURI DAY 11: KANCHANABURI / RATCHABURI / HUA HIN DAY 12: HUA HIN DAY 13: HUA HIN / BANGKOK DAY 14: BANGKOK 3 | P a g e ITINERARY DETAIL Day 1 : Monday 00.00.2012 – BANGKOK (D) TVIG: Meet and Greet your PRIVATE INTERNATIONAL TOURLEADER at the international airport Suvarnabhumi, in Bangkok - http://www.suvarnabhumiairport.com/index_en.php TVIG: Services of a PRIVATE INTERNATIONAL TOURLEADER (English-speaking/available from 08:00am until 18:00pm) TRIO: Transfer In – private airco vehicle – (Bangkok Airport) to (Bangkok Hotel) – 30Km / 19Mi – 00 Hrs 35’ INFO: Bangkok (Thai:’Krung Thep’) is the capital, largest urban area and primary city of Thailand. Known in Thai as “Krung Thep Maha Nakhon”, meaning "city of angels" for short, it was a small trading post at the mouth of the Chao Phraya River during the Ayutthaya Kingdom. It came to the forefront of Siam when it was given the status as the capital city in 1768 after the burning of Ayutthaya. -

July 8Th, 2016)
PROGRAM (First Version) DAY 1 (July 7th, 2016) DAY 2 (July 8th, 2016) Registration 8: Registration 9:00-10:30 Session A1, B1 9:00-10:15 Sessions A2, B2, C2, Poster 2 9:00-10:15 Session C1 (Day 10:30-10:50 Opening Address* & Tea reception 10:15-10:35 Tea break s 10:50-11:50 D1, E1, F1, Poster 1 10:35-11:50 Sessions D2, E2, F2 1 - 3 2 11:50-13:00 Lunch (Restaurant on 2/F) 11:50-13:00 Lunch (Restaurant on 2/F) 0 ) - 16: 13:00-15:15 Sessions G1, H1, J1 13:00-15:15 Sessions G2, H2, J2 00 15:15-15:35 Tea break 15:15-15:35 Tea break 15:35-17:45 Sessions K1, L1, M1 15:35-17:45 T.B.A. * Opening Address: Associate Professor Dr. Tanida Phatisena, Director of Research and Development Institute, Nakhon Rajasima Rajabhat University, Thailand (venue: Kujyaku, 2/F). Session A1: INTERNATIONAL TRADE & FINANCE (venue: Kaede, 2/F) Chair: Grzegorz Mazur (Poznan University of Economics and Business) The Impact of NAFTA on the Trade with Non-member Countries: the Case of Canadian Chinese Trade (s16-060) Speaker: Ying Kong (York University, Canada & Tsinghua University, China) The Impact of Financial and Trade Integration on Business Cycles in Emerging Markets (s16-093) Speaker: Lathaporn Ratanavararak (Chulalongkorn University) The Process of Banking Expansion in Asian Developing Countries: The Case of Nepal in Comparison with Japan (s16-114) Speaker: Shinichiro Maeda (Meijo University) Effects of Foreign Direct Investment on GDP Growth of Myanmar: Analysis for the Year of 1989-2014 (s16-170) Speaker: Ei Ei Phyu (Thammasat University) The European Union – Japan -

Download (786Kb)
This article has been published in a revised form in Journal of Southeast Asian Studies, 47 (3). pp. 366-392. https://doi.org/10.1017/S0022463416000242 This version is published under a Creative Commons CC-BY-NC-ND. No commercial re-distribution or re-use allowed. Derivative works cannot be distributed. © The National University of Singapore 2016 The case for proto-Dvāravatī: A review of the art historical and archaeological evidence Stephen A. Murphy The mid-first millennium CE represents a crucial period in the emergence of early polities in Southeast Asia. However, disagreement remains between archaeologists and art historians as to the precise dating of this shift from prehistory to history. This article focuses on the Dvāravatī period and re-evaluates evidence in Thai and Western language publications. A growing number of sites excavated over the past two decades in particular show occupation from c. the fourth to fifth century onwards while others provide a continual sequence stretching back well into the Iron Age. I argue that evidence from these sites makes a strong case for postulating a proto- Dvāravatī period spanning c. the fourth to fifth centuries. In doing so this article proposes this period as the timeframe within which the nascent traits and characteristics of what becomes Dvāravatī in the seventh to ninth centuries are present and gradually developing. The transition from prehistory to history in Mainland Southeast Asia has been a central issue of scholarship ever since the first attempts to explain the origins of its ‘classical states’ over a century ago. Ideas based around the ‘Indianisation’ paradigm saw this transition as happening over a short period of time based almost exclusively on external influences. -

Facility Location Problem
การพฒั นาตวั แบบการจดั สรรตา แหน่งของหน่วยการแพทย์ฉุกเฉิน เพอื่ ลดระยะเวลาการเข้าถงึ จุดเกดิ เหตุ กรณีศึกษาจังหวัดนครราชสีมา นายวโรรส อินทรศิริพงษ์ วทิ ยานิพนธ์นีเ้ ป็ นส่วนหนึ่งของการศึกษาตามหลกั สูตรปริญญาวศิ วกรรมศาสตรมหาบัณฑิต สาขาวชิ าวศิ วกรรมอุตสาหการ มหาวทิ ยาลัยเทคโนโลยสี ุรนารี ปีการศึกษา 2557 THE DEVELOPMENT OF AN EMS FACILITY LOCATION MODEL TO MINIMIZE RESPONSE TIME : A CASE STUDY OF NAKHON RATCHASIMA ROVINCE Waroros Intarasiripong A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Engineering in Industrial Engineering Suranaree University of Technology Academic Year 2014 การพฒั นาตัวแบบการจัดสรรต าแหน่งของหน่วยการแพทย์ฉุกเฉิน เพอื่ ลดระยะเวลาการเข้าถึงจุดเกดิ เหตุ กรณีศึกษาจังหวัดนครราชสีมา (ตัวอักษรเข้มขนาด 18) มหาวิทยาลัยเทคโนโลยีสุรนารี อนุมตั ิให้นบั วิทยานิพนธ์ฉบบั น้ีเป็นส่วนหน่ึงของการศึกษา ตามหลักสูตรปริญญามหาบัณฑิต คณะกรรมการสอบวิทยานิพนธ์ ____________________________ (รศ. ดร.พรศิริ จงกล) ประธานกรรมการ ____________________________ (ผศ. ดร.พงษ์ชัย จิตตะมัย) กรรมการ (อาจารย์ที่ปรึกษาวิทยานิพนธ์) ____________________________ (ผศ. ดร.ปภากร พิทยชวาล) กรรมการ ____________________________ ____________________________ (ศ. ดร.ชูกิจ ลิมปิจานง ค์) (รศ. ร.อ. ดร.กนต์ธร ชานิประศาสน์ ) รองอธิการบดีฝ่ายวิชาการและนวัตกรรม คณบดีสานักวิชาวิศวกรรมศาสตร์ วโรรส อินทรศิริพงษ์ : การพฒั นาตวั แบบการจดั สรรตา แหน่งของหน่วยการแพทย ์ ฉุกเฉินเพื่อลดระยะเวลาการเขา้ ถึงจุดเกิดเหตุ กรณีศึกษาจังหวัดนครราชสีมา (THE DEVELOPMENT OF AN EMS FACILITY LOCATION MODEL TO MINIMIZE RESPONSE TIME : A CASE STUDY OF NAKHON -

Working Conditions Thai HDD Industry
Working Conditions in Thailand's Hard Disk Drive Industry SOMO November 2010 Working Conditions in Thailand's Hard Disk Drive Industry Albert ten Kate & Esther de Haan Amsterdam, November 2010 SOMO is an independent research organisation. In 1973, SOMO was founded to provide civil society organizations with knowledge on the structure and organisation of multinationals by conducting independent research. SOMO has built up considerable expertise in among others the following areas: corporate accountability, financial and trade regulation and the position of developing countries regarding the financial industry and trade agreements. Furthermore, SOMO has built up knowledge of many different business fields by conducting sector studies. 2 Working Conditions in Thailand’s Hard Disk Drive Industry Colophon Working Conditions in Thailand’s Hard Disk Drive Industry November 2010 Authors: Albert ten Kate & Esther de Haan Cover design: Annelies Vlasblom ISBN: 978-90-71284-65-6 DOEN Foundation financed the formation of this publication, however DOEN Foundation is not the sender of this publication. Therefore DOEN Foundation is not responsible for the content or accountable for any damage as a result of wrong or incomplete information in this publication. Published by: Stichting Onderzoek Multinationale Ondernemingen Centre for Research on Multinational Corporations Sarphatistraat 30 1018 GL Amsterdam The Netherlands Tel: + 31 (20) 6391291 Fax: + 31 (20) 6391321 E-mail: [email protected] Website: www.somo.nl This document is licensed under the Creative -

Species Diversity of Aquatic Mollusks and Their Cercarial Infections; Khao Yai National Park, Thailand
J Trop Med Parasitol. 2012;35:37-47. ORIGINAL ARTICLE Available online at www.ptat.thaigov.net Species Diversity of Aquatic Mollusks and Their Cercarial Infections; Khao Yai National Park, Thailand Duangduen Krailas1, Sirilak Chotesaengsri1, Wivitchuta Dechruksa1 Suluck Namchote1, Chatapat Chuanprasit1, Nuanpan Veeravechsukij1 Dusit Boonmekam1, Tunyarut Koonchornboon2 1 Department of Biology, Faculty of Science, Silpakorn University, Nakhon Pathom; 2 Department of Anatomy, Phramongkutklao College of Medicine, Bangkok, Thailand Abstract quatic mollusks were investigated by opportunistic collection in Khao Yai National Park, A Thailand, every two months from March 2006 to January 2007. Our study focused on the habitat and species diversity of aquatic mollusks, which can act as intermediate hosts of parasitic trematodes. The study assembled baseline information on parasitic animal and human diseases in this area. Three sampling areas were investigated for mollusk diversity: Kongkaew Waterfall, Lam Takhong Stream, and Heo Suwat Waterfall. The mollusks were collected by hand-pick and scoop methods. Five species of gastropod and bivalve mollusks were found: Melanoides tuberculata, Filopaludina martensi martensi, Clea (Anentome) helena, Pseudodon mouhoti, and Corbicula javanica. The physicochemical characteristics and water quality of the streams varied slightly from one area to another, and between seasons. The water temperature was around 22-25 °C, and dissolved oxygen 7.1-9.5 mg/l. The cercarial infections of the mollusks collected were investigated by shedding and crushing methods, and were categorized into three species: Apatemon gracilis, Mesostephanus appendiculatus, and Loxogenoides bicolor. A. gracilis and M. appendiculatus cercariae were found in the snail C. helena, in Lam Takhong Stream and Heo Suwat Waterfall. -
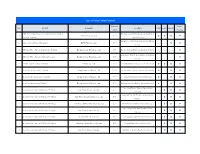
List of Solar Power Projects Capacity Public No
List of Solar Power Projects Capacity Public No. Project Company Location COP ESA IEE-SD (MW) Hearing 5 MW Photovoltaic Power Generation Project at Saraburi Tha Maprang Sub District, Kaeng Khoi District, 1 Infinite Green Co.,Ltd. 5.00 l l l l province, Thailand Saraburi Province Bang Pa-in District, Phra Nakhon Si Ayutthaya 2 Bangchak Solar Farm at Bangpa-In BCPG Public Co., Ltd. 38.00 - - l l Province 3 BSE Solar Power Plant at Chaiyaphum, Thailand Bangchak Solar Energy Co., Ltd. 8.00 Bamnet Narong District, Chaiyaphum Province - - - l Bang Pahan District, Phra Nakhon Si Ayutthaya 4 BSE Solar Power Plant at Ayutthaya,Thailand Bangchak Solar Energy Co., Ltd. 8.00 - - - l Province 5 EA Solar Farm at Lopburi, Thailand EA Solar Co., Ltd. 8.00 Phatthana Nikhom District, Lopburi Province l l l l 6 Solar Farm at Phitsanulok, Thailand Energy Absolute Public Co., Ltd. 90.00 Phrom Phiram District, Phitsanulok Province l - l l 7 Solar Farm at Nakhonsawan, Thailand Energy Absolute Public Co., Ltd. 90.00 Takhli District, Nakhon Sawan Province l - l l 8 Solar Farm at Lampang, Thailand Energy Absolute Public Co., Ltd. 90.00 Mueang Lampang District, Lampang Province l - l l Non Sung District, Nakhon Ratchasima 9 Solar Power Company 94 MW Solar PV Project Solar Power (Korat 1) Co.,Ltd. 6.00 - l l l Province Sawang Daen Din District, Sakon Nakhon 10 Solar Power Company 94 MW Solar PV Project Solar Power (Sakon Nakhon 1) Co.,Ltd. 6.00 - l l l Province 11 Solar Power Company 94 MW Solar PV Project Solar Power (Nakhon Phanom 1) Co.,Ltd. -

Buddhism and Its Relationship to Dvaravati Period Settlement Patterns and Material Culture in Northeast Thailand and Central Laos C
Buddhism and its Relationship to Dvaravati Period Settlement Patterns and Material Culture in Northeast Thailand and Central Laos c. Sixth–Eleventh Centuries A.D.: A Historical Ecology Approach to the Landscape of the Khorat Plateau STEPHEN A. MURPHY introduction The majority of previous studies on the development, nature, and spread of early Buddhism throughout Southeast Asia have focused on Buddhist art or archaeological sites. The art history approach primarily looks at issues concerning iconography, style, and influences from India and Sri Lanka, while the site-based archaeology approach usually examines religious architecture, monuments, material remains, and occupa- tional sequences of settlements without necessarily attempting to explain how the wider environment influenced these factors. Consequently, little consideration has yet been given to understanding Buddhism from the wider perspective of its location within the landscape and its relationship to settlement patterns throughout the region, as well as the pivotal role it played in the shaping and trajectory of different societies. Furthermore, Buddhist scholars have focused almost exclusively on textual and epi- graphic sources, which tend to be privileged over archaeological or art historical evi- dence, thus creating an inherent bias in understandings of this religion (e.g., Schopen 1997 : 1–22). These issues are addressed herein by focusing on the emergence and development of Buddhism in areas of northeast Thailand and the Vientiane and Savannakhet prov- inces of Central Laos during the Dvaravati period (sixth through eleventh centuries a.d.).1 Using this region and time frame as a case study, this article employs the theo- retical framework of historical ecology coupled with insights and methods derived from landscape archaeology.