1000000000 Reckitt Benckiser Treasury Services
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acquisition Agreement for Paras Pharmaceuticals -- Current
Dealdoc Acquisition agreement for Paras Pharmaceuticals Paras Pharmaceuticals Reckitt Benckiser Dec 13 2010 © 2009-2021, Wildwood Ventures Ltd. All rights reserved. Acquisition agreement for Paras Pharmaceuticals Paras Pharmaceuticals Companies: Reckitt Benckiser Announcement date: Dec 13 2010 Deal value, US$m: 730.0 : acquisition value • Details • Financials • Termsheet • Press Release • Filing Data • Contract Details Announcement date: Dec 13 2010 Pharmaceutical Industry sectors: Consumer health Financials Deal value, US$m: 730.0 : acquisition value Termsheet 13 December 2010 Reckitt Benckiser has agreed to buy Paras forINR 32.6 billion (Indian Rupees) (approximately GBP 460 million). RB will finance the transaction from existing facilities. Press Release RB Buys India’s Paras Pharmaceuticals Ltd 13 December 2010 Reckitt Benckiser Group plc (RB) today announces that it has agreed to buy Paras Pharmaceuticals Limited (Paras) forINR 32.6 billion (Indian Rupees) (approximately GBP 460 million) from the current shareholders, including the Patel family and Actis, the emerging markets private equity investor. RB will finance the transaction from existing facilities. Paras is a privately-owned Indian company with a portfolio of leading Indian over the counter Health and Personal Care brands including: Moov, the No 2 topical analgesic pain ointment, D’Cold, the No 2 cold & flu remedy, Dermicool, the No 2 for prickly heat, Krack, the No 1 medicated skin treatment for cracked heels and Itch Guard and Ring Guard anti fungal creams. In addition, Paras has a personal care business led by Set Wet, a leading hair gel and deodorant brand. In the fiscal year ending March 2010, Paras generated net sales of INR 4,014 million, representing a mid-teens average growth rate over the last four years, and operating EBITDA for the same year of INR 1,083 million (approximately GBP 56 million and GBP 15 million respectively). -

Consumer Superbrands 2019 Top 10 Consumer Superbrands Relevancy
Consumer Superbrands 2019 Top 10 Consumer Superbrands BRAND CATEGORY LEGO 1 Child Products - Toys and Education Apple 2 Technology - General Gillette 3 Toiletries - Men's Grooming Rolex 4 Watches British Airways 5 Travel - Airlines Coca-Cola 6 Drinks - Non-Alcoholic - Carbonated Soft Drinks Andrex 7 Household - Kitchen Rolls, Toilet Roll and Tissues Mastercard 8 Financial - General Visa 9 Financial - General Dyson 10 Household & Personal Care Appliances Relevancy Index Top 20 BRAND CATEGORY Amazon 1 Retail - Entertainment & Gifts Aldi 2 Retail - Food & Drink Macmillan Cancer Support 3 Charities Netflix 4 Media - TV Google 5 Social, Search & Comparison Sites Lidl 6 Retail - Food & Drink PayPal 7 Financial - General LEGO 8 Child Products - Toys and Education Samsung 9 Technology - General YouTube 10 Social, Search & Comparison Sites Visa 11 Financial - General Heathrow 12 Travel - Airports Purplebricks 13 Real Estate Cancer Research UK 14 Charities Oral-B 15 Toiletries - Oral Care Apple 16 Technology - General Dyson 17 Household & Personal Care Appliances TripAdvisor 18 Travel - Agents & Tour Operators Nike 19 Sportswear & Equipment Disney 20 Child Products - Toys and Education continues... Consumer Superbrands 2019 Category Winners CATEGORY BRAND Automotive - Products Michelin Automotive - Services AA Automotive - Vehicle Manufacturer Mercedes-Benz Charities Cancer Research UK Child Products - Buggies, Seats and Cots Mamas & Papas Child Products - General JOHNSON'S Child Products - Toys and Education LEGO Drinks - Alcoholic - Beer, Ale -

Reckitt Partners with the Cambridge Centre for Risk Studies As It Advances Its Commitments to Carbon Neutrality
Reckitt partners with the Cambridge Centre for Risk Studies as it advances its commitments to carbon neutrality 9 June 2021 – Reckitt today announces its strategic partnership with the Cambridge Centre for Risk Studies (CCRS) as it progresses its sustainability ambitions. The partnership advances Reckitt’s ongoing commitment to deliver on the Paris Accord and its ambition for carbon neutrality by 2040 while delivering growth and long-term shareholder value. Its strategy for managing climate change includes action across the entire footprint of its organisation. The CCRS is working with Reckitt to support its climate change activity with analytics, including: • A detailed assessment of leading climate science to test and inform activity within changing patterns of extreme weather, and different scenarios of transition risks including regulatory change and consumer sentiment trends. • Strengthening Reckitt’s financial quantification of these impacts, by mapping Reckitt’s global business activities and the potential consequences of future changes. • Analysis of potential ways to evolve the business to achieve its goals for the Paris Accord and net-zero ambitions. Reckitt is the official Hygiene Partner for COP26, and is playing its part in tackling climate change by setting science-based targets to reach net zero emissions. In June 2020, Reckitt committed to accelerate the delivery of the Paris Climate Change Agreement and RE100, with the goal of achieving 100% renewable electricity across its operations by 2030 as part of an ambition to be carbon neutral by 2040. David Croft, Global Director of Sustainability, Reckitt, said: “We selected Cambridge because of its experienced, comprehensive and science-based approach to assessing and addressing climate-related risks, opportunities and metrics. -

RB Partners with Veolia to Drive a Circular Plastics Economy
RB partners with Veolia to drive a circular plastics economy Reckitt Benckiser (RB) and Veolia have today announced their partnership to drive the shift to a circular plastics economy. The partnership with Veolia is another step RB is taking to fulfil its pledge to make 100 percent of its plastic packaging recyclable and to contain at least 25 percent recycled content by 2025. Veolia and RB started working together two years ago to work on increasing the use of postconsumer recycled plastic in RB’s packaging. The first offering from the partnership is the new packaging for Finish Quantum. The packaging now contains 30% recycled plastic and is proudly grey in colour because RB has decided not to add masking pigments or additives to the packaging. The companies will continue their collaboration on designing for recyclability and the maximization of post-consumer recycled content. To accelerate the circular plastics economy, a team of 20 experts from RB and Veolia are also working to develop enhanced collection systems, driving behaviour change to aid consumer sorting habits and improving recycling from households. “The partnership between Veolia and RB Hygiene Home brings together our complementary capabilities to drive a positive contribution to the circular economy. We have just started the journey by increasing recycled content and improving recyclability and are excited about the wider opportunities across our value chains”, says Fabrice Beaulieu, EVP Marketing, R&D and Sustainability for RB Hygiene Home. “We are delighted to be collaborating with RB on this ongoing strategy to reduce their environmental footprint. We are working hand-in-hand with RB to develop packaging with greater recycled content and improved recyclability for the RB group’s consumers,” says Antoine Frérot, Chairman and CEO of Veolia. -

COVID-19 Public Policy Group Report April 25, 2020
COVID-19 Public Policy Group Report April 25, 2020 President Donald Trump on Friday signed the US$484 Tax and Economic Development Updates billion Paycheck Protection Program and Health Care Enhancement Act into law, triggering a joint announcement With the interim PPP funding bill now signed into law, the by the U.S. Department of the Treasury and Small Business SBA and Treasury Department have indicated that applications Administration (SBA) that the CARES Act‘s Paycheck will be accepted again beginning at 10:30am on Monday, April Protection Program (PPP) will resume operations Monday 27. In a joint statement, Treasury Secretary Mnuchin and SBA morning at 10:30am EST. Meanwhile, details continued to Administrator Jovita Carranza noted: “The PPP has supported trickle out about the respective agendas of the White House more than 1.66 million small businesses and protected and Democratic congressional leadership for negotiations over 30 million jobs for hardworking Americans…With the over the next federal COVID-19 response measure. additional funds appropriated by Congress, tens of millions of additional workers will benefit from this critical relief.” The House Speaker Nancy Pelosi (D-CA) and her lieutenants will two also made clear that the SBA “has properly coded the have the pen in writing the first iteration of the next pandemic system to account for changes,” including the US$60 billion response bill, which House Majority Leader Steny Hoyer (D- set aside for smaller financial institutions. Additionally, the MD) has indicated will be unveiled by May 4. SBA yesterday published additional PPP guidance regarding Congressional Democrats are reportedly eyeing the inclusion requirements for promissory notes, authorizations, affiliation, of another round of direct “stimulus” cash payments to and eligibility. -

Product & Price# of Barcodes
Product & Price# of Barcodes <span class="hilite">Cleaning</span> Air Wick Freshmatic Ultra1 Starter Kit (2 gadgets + 2 refills) - $12.99 Air Wick Freshmatic Ultra4 Refill (4 pack/6.17 oz) - $13.99 Air Wick Scented Oils1 (1 warmer + 7 refills) - $11.99 Air Wick Scented Oils1 Refill (8 pack) - $12.99 Air Wick Candles (twin2 pack) - $9.99 Clorox Foaming Bathroom3 Cleaner (3 pack/30 oz) - $8.49 Clorox Toilet Wand (361 ct refill with bonus wand) - $16.49 Clorox Automatic Toilet1 Bowl Cleaner (6 ct/3.5 oz) - $10.79 Clorox Disinfecting Wipes1 (5 pack/78 ct) - $14.99 Clorox Green Works Natural2 Cleaner (twin pack) - $4.99 Clorox Oxi Magic (7.81 lbs) - $9.99 Clorox Clean Up (1802 oz + 32 oz trigger) - $10.49 Clorox Pool & Spa Shock1 Plus, 12 pk./1 lb. $34.99 Clorox Pool & Spa Algaecide and Clarifier, 2 pk./1 gal. $14.99 Clorox Pump 'n Clean (2 pumps + 2 refills/18 oz) - $9.99 Clorox Bleach (2/182 2oz bottles) - $5.99 Damp Rid Moisture 46 Pack Hanging - $9.99 DampRid Buckets (6 pack)1 - $9.99 Fabuloso All Purpose 3Cleaner (169 oz) - $7.99 Febreze Air Effects (32 pack/9.7 oz) - $6.99 Febreze Air Effects Heavy1 Duty Crisp Clean Air Freshener (twin pack/19.4 oz) - $5.99 Febreze Set and Refresh2 (2 ct) - $7.99 Febreze Fabric 2 ct. 541 oz. - $7.99 Febreze Car Vent Clips3 (4 ct) $8.99 Febreze Unstopables2 (3 pack/8.8 oz) 8.99 Formula 409 (180 oz 1+ 32 oz) - $11.99 Glade PlugIns (2 warmers1 + 6 refills) - $11.49 Glade PlugIns (8 pack2 refill) - $12.99 Kaboom (twin pack/403 oz) - $8.79 Liquid Plumbr Foam Pipesnake2 (3 pack/17 oz) - $8.99 Liquid Plumbr ProStrength3 (twin pack/80 oz) - $9.99 Lysol Neutra Air (3 pack/164 oz) - $9.99 Lysol Neutra Air Sanitizing4 Spray, 3 pk./16 oz. -

Rb-Annual-Report-2012.Pdf
Reckitt Benckiser Group plc Reckitt Benckiser Group Healthier Happier Annual Report and Financial Statements 2012 Stronger Reckitt Benckiser Group plc Annual Report and Financial Statements 2012 Contents 1 Chairman’s Statement 2 Chief Executive’s Statement 10 Business Review 2012 18 Board of Directors and Executive Committee 19 Report of the Directors 22 Chairman’s Statement on Corporate Governance 24 Corporate Governance Report 30 Statement of Directors’ Responsibilities 31 Directors’ Remuneration Report 38 Independent Auditors’ Report to the members of Reckitt Benckiser Group plc 39 Group income statement 39 Group statement of comprehensive income 40 Group balance sheet 41 Group statement of changes in equity 42 Group cash flow statement 43 Notes to the financial statements 75 Five-year summary 76 Parent Company – Independent Auditors’ Report to the members of Reckitt Benckiser Group plc 77 Parent Company balance sheet 78 Notes to the Parent Company financial statements 84 Shareholder information Chairman’s Statement largest consumer health care category in The Board conducted its regular reviews the world with the acquisition of Schiff of the Company’s brands, geographic area Nutrition International, Inc. (Schiff) and and functional performance together with its leading US brands in the vitamins, detailed reviews of its human resources. minerals and supplements market. There The Board also completed its annual were also a few disposals of non core assessment of corporate governance assets. Net debt at the end of 2012, after including Board performance, corporate paying for dividends, net acquisitions and responsibility, and reputational and organisation restructuring, stood at business risk. £2,426m (2011: £1,795m). AGM Resolutions Your Board proposes an increase in the final The resolutions, which will be voted dividend of +11%, taking it to 78p per upon at our AGM of 2 May 2013 are share, and bringing the total dividend for fully explained in the Notice of Meeting. -

RB Acquires Queen V
RB Acquires Queen V An insurgent consumer loved brand in $7Bln+ female intimate hygiene category, re- enforcing commitment to Sexual Wellbeing growth, de-stigmatization and development. London, January 20, 2021 – RB today announces that it has acquired Queen V, a feminine wellness brand established in the US, focused on vaginal health. Founded in 2018 in California, Queen V takes a unique and inclusive approach to vaginal health with its commitment to make feminine wellness more accessible and empower women to take control of their bodies. The acquisition of the Queen V brand is demonstrative of RB’s commitment to innovative, purpose-driven brands that consumers love and is in line with the strategy to play in new spaces and adjacencies. Queen V will be part of RB’s Health Global Business Unit, alongside leading sexual wellbeing brands, KY and Durex. “Queen V is a fantastic purpose-driven brand with products that appeal to the needs of our diverse and evolving customer base. This innovative brand has the potential to enhance wellness and make a positive difference to many consumers’ daily lives. Working together with the Queen V team, we are committed to the shared mission of de-stigmatization, focusing on women’s needs and vagina-positivity” said Olga Osminkina-Jones, Global Senior Vice President of Sexual Wellbeing at RB. For further information, please contact: [email protected] / [email protected] About RB RB* is driven by its purpose to protect, heal and nurture in a relentless pursuit of a cleaner, healthier world. We fight to make access to the highest-quality hygiene, wellness and nourishment a right, not a privilege, for everyone. -
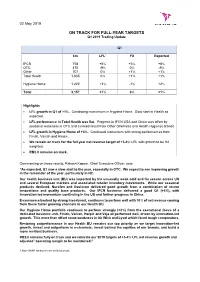
ON TRACK for FULL-YEAR TARGETS Q1 2019 Trading Update
02 May 2019 ON TRACK FOR FULL-YEAR TARGETS Q1 2019 Trading Update Q1 £m LFL1 FX Reported IFCN 758 +5% +3% +8% OTC 470 -9% 0% -9% Other 707 0% +1% +1% Total Health 1,935 0% +1% +1% Hygiene Home 1,222 +3% -1% +2% Total 3,157 +1% 0% +1% Highlights LFL growth in Q1 of +1%. Continuing momentum in Hygiene Home. Slow start in Health as expected. LFL performance in Total Health was flat. Progress in IFCN USA and China was offset by seasonal weakness in OTC and a mixed result from Other (Wellness and Health Hygiene) brands. LFL growth in Hygiene Home of +3%. Continued momentum with strong performances from Finish, Vanish and Harpic. We remain on track for the full year net revenue target of +3-4% LFL with growth to be H2 weighted. RB2.0 remains on track. Commenting on these results, Rakesh Kapoor, Chief Executive Officer, said: “As expected, Q1 saw a slow start to the year, especially in OTC. We expect to see improving growth in the remainder of the year, particularly in H2. Our health business unit (BU) was impacted by the unusually weak cold and flu season across US and several European markets and associated retailer inventory movements. While our seasonal products declined, Nurofen and Gaviscon delivered good growth from a combination of recent innovations and quality base products. Our IFCN business delivered a good Q1 (+5%), with innovation led momentum continuing in the US and further progress in China. E-commerce backed by strong investment, continues to perform well with 10% of net revenue coming from these faster growing channels in our Health BU. -

Sexing Capitalism: Condoms and Industrial Change Peter Chua, San Jose State University
San Jose State University From the SelectedWorks of Peter Chua August, 2004 Sexing Capitalism: Condoms And Industrial Change Peter Chua, San Jose State University Available at: https://works.bepress.com/peter_chua/17/ Sexing Capitalism: Condoms And Industrial Change Peter Chua Department of Sociology San José State University [email protected] Paper Submitted for the 2003 99th Annual Meeting of the American Sociological Association, San Francisco, CA Sexing Capitalism: Condoms And Industrial Change Abstract In the late 1700s, condoms were luxury items for the affluent in Western Europe, but by the 1970s, the US government gave free condoms out to poor women in Third World areas. Moreover condom availability has increased dramatically since the global emergence of the AIDS pandemic in the 1980s, adding to the already fervent social stigmatization and political contentions on morality, sexuality, and wellbeing that condom use brings. This paper focuses on the strategically joint-relationship between manufacturing firms and governments to foster distinct profit-oriented condom social relations and moral-symbolic regimes of sexual cultures. Proposing a sex-situated theory of capitalist firms, the paper examines the crucial social aspect of condom production, focusing on the changes in the condom manufacturing industry from its initial colonial- “warfare” period (1880s-1930s), its period of massive welfare-state expansion (1940s- 1970s), and its recent neoliberal consolidation (1980s-2000s). Keywords Capitalist Institutions, Sexual Cultures, Condom Commodities; Social Aspects of Production; Economic Neoliberalism Sexing Capitalism: Condoms And Industrial Change In the late 1700s, condoms were luxury items for the affluent in Western Europe, but by the 1970s, the US government gave free condoms out to poor women in Third World areas. -

Northern Ireland Prescription Code Book Drugs Section September 2021
Family Practitioner Services, Pharmaceutical Services, 2 Franklin Street, Belfast BT2 8DQ Telephone No. 028 9053 5613, Fax No. 028 9053 2963 Northern Ireland Prescription Code Book Drugs section September 2021 Page 1 of 587 Effective for prescriptions dispensed September 2021 BSO Code dm+d Pack Description Code Number Special ZD Of Container 38960 4SURE beta-ketone testing strips 4S-810-4183401-001 (Nipro Diagnostics (UK) Ltd) 10 strip strips 38961 4SURE testing strips (Nipro Diagnostics (UK) Ltd) 50 strip strips 4144 AAA 1.5mg/dose sore throat spray (Manx Healthcare Ltd) 60 dose [BSO pack = 1] BSO Unit Of Measure Code No. devices dispensed 70598 (DT) Abacavir 600mg / Lamivudine 300mg tablets 30 tablet tablets 9154 Abasaglar 100units/ml solution for injection 3ml cartridges (Eli Lilly and Company Ltd) 5 cartridge cartridges ZD 9038 Abasaglar KwikPen 100units/ml solution for injection 3ml pre-filled pens (Eli Lilly and Company Ltd) 5 pre- injections ZD filled disposable injection 70088 (DT) Abatacept 125mg/1ml solution for injection pre-filled disposable devices 4 pre-filled disposable injections ZD injection 70089 (DT) Abatacept 125mg/1ml solution for injection pre-filled syringes 4 pre-filled disposable injection injections ZD 70144 Abelcet 100mg/20ml concentrate for suspension for infusion vials (Teva UK Ltd) 10 vial vials Hospital Only 3303 Abidec Multivitamin drops (Omega Pharma Ltd) 25 ml mls 4043 Abilify 10mg orodispersible tablets (Otsuka Pharmaceuticals (U.K.) Ltd) 28 tablet 4 x 7 tablets tablets 3006 Abilify 10mg tablets (Otsuka -

Elegant Napkins 500Ct 12Pk $22.49 Elegant Tissues 230Ct 24Pk $20.49
1 2 Elegant Napkins Elegant Tissues 500ct 12pk $22.49 230ct 24pk $20.49 For questions regarding prices on Terms and Conditions large quantity orders, please email $1,000 minimum order for free delivery within the 5 boroughs of NY, or fax us a list of the items you are NJ & PA. interested in along with quantities. Tailgate delivery only. We can order specialty items Freight charge of $75 for orders under minimum. direct from all major Call for rates outside the NJ/NY area. manufacturers. Easy ordering: All sales are COD cash unless arranged in advanced. Phone, Fax, Online or contact your We ship paper products or specials only equal amount of the other sales rep. We appreciate your products. business and look forward to Prices and availability are subject to change. providing you with quality products Unit price is listed for reference only. and excellent customer service. Prices are by the case ONLY. We are not responsible for any typographical errors. Pictures are a representation of product and may not be exactly the same. We reserve the right to limit quantities ordered. 1 2 3 Member's Mark Long Member's Mark Member's Mark Long Pepsi-Cola Can 4 Grain White Rice - 25lb Parboiled Rice Long Grain White Rice - 50lb 12oz/36pk Grain - 25lb Clorox Toilet Bowl Cleaner All Soft Scrub Cleaner All kinds- kinds 24oz/9pk 24oz/12pk $22.49 $21.49 $11.59 $13.49 $19.49 $0.35 $1.79 $2.50 $11.99 $11.59 $13.99 $13.49 $19.99 $19.49 $12.99 $12.59 Pledge Multi-Surface Spray All Gain Ultra Powder Detergent All Pepsi-Cola Bottles Coca Cola Cans