Eptatretus Stoutii)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stomach Content Analysis of Short-Finned Pilot Whales
f MARCH 1986 STOMACH CONTENT ANALYSIS OF SHORT-FINNED PILOT WHALES h (Globicephala macrorhynchus) AND NORTHERN ELEPHANT SEALS (Mirounga angustirostris) FROM THE SOUTHERN CALIFORNIA BIGHT by Elizabeth S. Hacker ADMINISTRATIVE REPORT LJ-86-08C f This Administrative Report is issued as an informal document to ensure prompt dissemination of preliminary results, interim reports and special studies. We recommend that it not be abstracted or cited. STOMACH CONTENT ANALYSIS OF SHORT-FINNED PILOT WHALES (GLOBICEPHALA MACRORHYNCHUS) AND NORTHERN ELEPHANT SEALS (MIROUNGA ANGUSTIROSTRIS) FROM THE SOUTHERN CALIFORNIA BIGHT Elizabeth S. Hacker College of Oceanography Oregon State University Corvallis, Oregon 97331 March 1986 S H i I , LIBRARY >66 MAR 0 2 2007 ‘ National uooarac & Atmospheric Administration U.S. Dept, of Commerce This report was prepared by Elizabeth S. Hacker under contract No. 84-ABA-02592 for the National Marine Fisheries Service, Southwest Fisheries Center, La Jolla, California. The statements, findings, conclusions and recommendations herein are those of the author and do not necessarily reflect the views of the National Marine Fisheries Service. Charles W. Oliver of the Southwest Fisheries Center served as Contract Officer's Technical Representative for this contract. ADMINISTRATIVE REPORT LJ-86-08C CONTENTS PAGE INTRODUCTION.................. 1 METHODS....................... 2 Sample Collection........ 2 Sample Identification.... 2 Sample Analysis.......... 3 RESULTS....................... 3 Globicephala macrorhynchus 3 Mirounga angustirostris... 4 DISCUSSION.................... 6 ACKNOWLEDGEMENTS.............. 11 REFERENCES.............. 12 i LIST OF TABLES TABLE PAGE 1 Collection data for Globicephala macrorhynchus examined from the Southern California Bight........ 19 2 Collection data for Mirounga angustirostris examined from the Southern California Bight........ 20 3 Stomach contents of Globicephala macrorhynchus examined from the Southern California Bight....... -

Lamprey, Hagfish
Agnatha - Lamprey, Kingdom: Animalia Phylum: Chordata Super Class: Agnatha Hagfish Agnatha are jawless fish. Lampreys and hagfish are in this class. Members of the agnatha class are probably the earliest vertebrates. Scientists have found fossils of agnathan species from the late Cambrian Period that occurred 500 million years ago. Members of this class of fish don't have paired fins or a stomach. Adults and larvae have a notochord. A notochord is a flexible rod-like cord of cells that provides the main support for the body of an organism during its embryonic stage. A notochord is found in all chordates. Most agnathans have a skeleton made of cartilage and seven or more paired gill pockets. They have a light sensitive pineal eye. A pineal eye is a third eye in front of the pineal gland. Fertilization of eggs takes place outside the body. The lamprey looks like an eel, but it has a jawless sucking mouth that it attaches to a fish. It is a parasite and sucks tissue and fluids out of the fish it is attached to. The lamprey's mouth has a ring of cartilage that supports it and rows of horny teeth that it uses to latch on to a fish. Lampreys are found in temperate rivers and coastal seas and can range in size from 5 to 40 inches. Lampreys begin their lives as freshwater larvae. In the larval stage, lamprey usually are found on muddy river and lake bottoms where they filter feed on microorganisms. The larval stage can last as long as seven years! At the end of the larval state, the lamprey changes into an eel- like creature that swims and usually attaches itself to a fish. -

Reporting for the Period from May 2015-April 2016
Washington Contribution to the 2016 Meeting of the Technical Sub-Committee (TSC) of the Canada-U.S. Groundfish Committee: Reporting for the period from May 2015-April 2016 April 26th-27th, 2016 Edited by: Dayv Lowry Contributions by: Dayv Lowry Robert Pacunski Lorna Wargo Mike Burger Taylor Frierson Todd Sandell Jen Blaine Brad Speidel Larry LeClair Phil Weyland Donna Downs Theresa Tsou Washington Department of Fish and Wildlife April 2016 Contents I. Agency Overview.....................................................................................................................3 II. Surveys.....................................................................................................................................4 III. Reserves..............................................................................................................................16 IV. Review of Agency Groundfish Research, Assessment, and Management.........................16 A. Hagfish............................................................................................................................16 B. North Pacific Spiny Dogfish and other sharks................................................................20 C. Skates..............................................................................................................................20 D. Pacific Cod......................................................................................................................20 E. Walleye Pollock..............................................................................................................21 -

Evaluation of O2 Uptake in Pacific Hagfish Refutes a Major Respiratory Role for the Skin Alexander M
© 2016. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2016) 219, 2814-2818 doi:10.1242/jeb.141598 SHORT COMMUNICATION It’s all in the gills: evaluation of O2 uptake in Pacific hagfish refutes a major respiratory role for the skin Alexander M. Clifford1,2,*, Alex M. Zimmer2,3, Chris M. Wood2,3 and Greg G. Goss1,2 ABSTRACT hagfishes, citing the impracticality for O2 exchange across the – Hagfish skin has been reported as an important site for ammonia 70 100 µm epidermal layer, perfusion of capillaries with arterial excretion and as the major site of systemic oxygen acquisition. blood of high PO2 and the impact of skin boundary layers on diffusion. However, whether cutaneous O2 uptake is the dominant route of uptake remains under debate; all evidence supporting this Here, we used custom-designed respirometry chambers to isolate hypothesis has been derived using indirect measurements. Here, anterior (branchial+cutaneous) and posterior (cutaneous) regions of we used partitioned chambers and direct measurements of oxygen Pacific hagfish [Eptatretus stoutii (Lockington 1878)] to partition whole-animal Ṁ and ammonia excretion (J ). Exercise consumption and ammonia excretion to quantify cutaneous and O2 ̇ Amm branchial exchanges in Pacific hagfish (Eptatretus stoutii) at rest and typically leads to increases in MO2 and JAmm during post-exercise following exhaustive exercise. Hagfish primarily relied on the gills for recovery; therefore, we employed exhaustive exercise to determine the relative contribution of the gills and skin to elevations in both O2 uptake (81.0%) and ammonia excretion (70.7%). Following metabolic demand. Given that skin is proposed as the primary site exercise, both O2 uptake and ammonia excretion increased, but only ̇ across the gill; cutaneous exchange was not increased. -

January 2015 • Vol
Published by the American Physiological Society – Integrating the Life Sciences from Molecule to Organism THEPHYSIOLOGIST January 2015 • Vol. 58/No. 1 A Matter of Opinion from the President What Does Peer Review Need? Research funding decisions require a strong peer review process. After all, who but the experts can identify the Guyton Educator of the Year Award most promising science? At the same time, complaining about peer review Herbert F. Janssen has always been one of scientists’ favorite pastimes. Although these complaints are not new, I believe our The humble gratitude I feel as recipient of current peer review system for grants is the Guyton Educator of the Year Award facing serious challenges. necessitates that I acknowledge my friend and colleague David Osborne for the nomination, Full Participation the colleagues and students who supported A major problem is that too few the nomination, and the numerous students senior, experienced reviewers choose who, over the years, helped develop my to serve on peer review panels. It is teaching style and philosophy of education. easy to blame the difficult funding I must also thank the American Physiological Society, the APS Selection Committee, and Continued on page 13 Herbert F. Janssen Elsevier for recognizing the importance of educators in APS. THIS ISSUE: Over a century ago, the Flexner Report concluded that didactic lectures 3 Success in Research at an were hopelessly antiquated and belonged to an age of accepted Undergraduate Institution dogma, “when the professor ‘knew’ and the students ‘learned’” (1). Abraham Flexner encouraged medical educators to provide students 17 Nebraska Physiological the opportunity to learn rather than be taught. -

Jawless Fishes of the World
Jawless Fishes of the World Jawless Fishes of the World: Volume 1 Edited by Alexei Orlov and Richard Beamish Jawless Fishes of the World: Volume 1 Edited by Alexei Orlov and Richard Beamish This book first published 2016 Cambridge Scholars Publishing Lady Stephenson Library, Newcastle upon Tyne, NE6 2PA, UK British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Copyright © 2016 by Alexei Orlov, Richard Beamish and contributors All rights for this book reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner. ISBN (10): 1-4438-8582-7 ISBN (13): 978-1-4438-8582-9 TABLE OF CONTENTS Volume 1 Preface ........................................................................................................ ix M. Docker Part 1: Evolution, Phylogeny, Diversity, and Taxonomy Chapter One ................................................................................................. 2 Molecular Evolution in the Lamprey Genomes and Its Relevance to the Timing of Whole Genome Duplications T. Manousaki, H. Qiu, M. Noro, F. Hildebrand, A. Meyer and S. Kuraku Chapter Two .............................................................................................. 17 Molecular Phylogeny and Speciation of East Asian Lampreys (genus Lethenteron) with reference to their Life-History Diversification Y. Yamazaki and -

THE PINNIPEDS of the CALIFORNIA CURRENT California
ANTONELIS AND FISCUS: PINNIPEDS OF THE CALIFORNIA CURRENT CalCOFI Rep., Vol. XXI, 1980 THE PINNIPEDS OF THE CALIFORNIA CURRENT GEORGE A. ANTONELIS. JR. AND CLIFFORD H. FISCUS Marine Mammal Division Northwest and Alaska Fisheries Center National Marine Fisheries Service National Oceanic and Atmospheric Administration 7600 Sand Point Way, N.E. Seattle, WA 981 15 ABSTRACT 10s pequenos peces en 10s cardumenes y peces ana- There are six species of pinnipeds-California sea dromos. Los dos focidos, otra vez con ciertas excep- lion, Zalophus californianus; northern sea lion, Eume- ciones, predan especies diferentes. Aparentemente, el topias jubatus; northern fur seal, Callorhinus ursinus; elefante marino se alimenta en aguas mas profundas que Guadalupe fur seal, Arctocephalus townsendi; harbor la foca peluda, alimentindose de especies demersales seal, Phoca uitulina richardsi; and northern elephant y benticas, y la foca peluda se alimenta de especiesdemer- seal, Mirounga angustirostris-that inhabit the study sales costeras y neriticas, entrando ocasionalmente en rios area of the California Cooperative Oceanic Fisheries y aguas estuarinas haciendopresa de 10s peces anadromos Investigations (CalCOFI). y otros pequeiios peces que entran regularmente en estas The numbers of animals in each population are given; aguas. the size, distribution, and seasonal movements are de- scribed. The known prey species of the pinnipeds are INTRODUCTION listed for each species. The otariids, with certain excep- The California Current, its components, and the Cali- tions, consume the same kinds of prey, although in slight- fornia Cooperative Oceanic Fisheries Investigations ly different amounts. In general they feed most commonly (CalCOFI) station plan have been described many times on the smaller schooling fishes and squids of the epi- in the past and are well known (Kramer et al. -

Characterizing the Deep: Surveys in the Monterey Bay National Marine Sanctuary 2007-2010 Acknowledgments
Characterizing the Deep: Surveys in the Monterey Bay National Marine Sanctuary 2007-2010 Acknowledgments enerous support for this project comes from the Monterey Bay Many thanks also to the Seafloor Mapping Lab at CSUMB, the US GNational Marine Sanctuary as well as California State University Geological Survey, and Monterey Bay Aquarium Research Institute for Monterey Bay’s Undergraduate Research Opportunities Center and the topographic maps of the seafloor throughout the study areas. James W. Rote Distinguished Professorship in Marine Science and Policy. All photos in this document (unless otherwise noted) were taken with This publication was conceived and implemented by the faculty, the ROV Beagle in the Monterey Bay National Marine Sanctuary. Credit staff and students of the Institute for Applied Marine Ecology at is to: IfAME/CSUMB/MBNMS/NOAA/MARE CSU Monterey Bay… Topside operations photos credit to Adam Alfasso, Ashley Knight, Heather Dr. James Lindholm – Founder & Director Kramp, Andrew DeVogelaere, Jason Adelaars Ashley Knight – Research Technician Jason Adelaars – Graduate Research Intern Special thanks to Robert Lea, Jean de Marignac, Donna Kline, and Bryon Megan Kelly – Graduate Research Assistant Downey for help with species identifications. Also many thanks to Heather Kramp – Undergraduate Research Assistant Judy Anderson (A Graphic Design Studio) for her expertise, talent, and patience with us in designing this report. ...with key support from Dr. Andrew DeVogelaere, Research Coordinator for the Monterey Bay National Marine -

Guide to the Coastal Marine Fishes of California
STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 157 GUIDE TO THE COASTAL MARINE FISHES OF CALIFORNIA by DANIEL J. MILLER and ROBERT N. LEA Marine Resources Region 1972 ABSTRACT This is a comprehensive identification guide encompassing all shallow marine fishes within California waters. Geographic range limits, maximum size, depth range, a brief color description, and some meristic counts including, if available: fin ray counts, lateral line pores, lateral line scales, gill rakers, and vertebrae are given. Body proportions and shapes are used in the keys and a state- ment concerning the rarity or commonness in California is given for each species. In all, 554 species are described. Three of these have not been re- corded or confirmed as occurring in California waters but are included since they are apt to appear. The remainder have been recorded as occurring in an area between the Mexican and Oregon borders and offshore to at least 50 miles. Five of California species as yet have not been named or described, and ichthyologists studying these new forms have given information on identification to enable inclusion here. A dichotomous key to 144 families includes an outline figure of a repre- sentative for all but two families. Keys are presented for all larger families, and diagnostic features are pointed out on most of the figures. Illustrations are presented for all but eight species. Of the 554 species, 439 are found primarily in depths less than 400 ft., 48 are meso- or bathypelagic species, and 67 are deepwater bottom dwelling forms rarely taken in less than 400 ft. -

The Biology of Hagfishes the Biology of Hagfishes
THE BIOLOGY OF HAGFISHES THE BIOLOGY OF HAGFISHES J0RGEN M0RUP J0RGENSEN, JENS PETER LOMHOLT, ROY E. WEBER and HANSMALTE Department of Zoophysiology Institute of Biological Sciences University of Aarhus Denmark luni Springer-Science+Business Media, B.V. Published by Chapman and HaU, an imprint of Thomson Science, 2--6 Boundary Row, London SEl 8HN, UK Thomson Science, 2-6 Boundary Row, London SEl 8HN, UK Thomson Science, 115 Fifth Avenue, New York, NY 10003, USA Thomson Science, Suite 750, 400 Market Street, Philadelphia, PA 19106, USA Thomson Science, Pappelallee 3, 694469 Weinheim, Germany First edition 1998 © 1998 Springer Science+ Business Media Dordrecht OriginaUy published by Chapman & HaU Ltdin 1998 Softcover reprint of the hardcover 1st edition 1998 Typeset in 10112pt Palatino by Cambrian Typesetters, Frimley, Surrey ISBN 978-94-010-6465-1 ISBN 978-94-011-5834-3 (eBook) DOI 10.1007/978-94-011-5834-3 AII rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, e1e~tronic, mechanical, photocopying, recording or otherwise, without the prior written permis sion of the publishers. Applications for permission shou1d be addressed to the rights manager at the London address of the publisher. The publisher makes no representation, express or imp1ied, with regard to the accuracy of the information contained in this book and cannot accept any legal responsibility or liability for any errors or omissions that may be made. A catalogue record for this book is available from the British Library Library ofCongress Catalog Card Number: 97-69524 8 Printed on acid-free text paper, manufactured in accordance with ANSIINISO Z39.48-1992 (Permanence of Paper). -
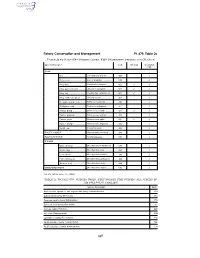
Species Codes: Fmp Prohibited Species and Cr Crab
Fishery Conservation and Management Pt. 679, Table 2c TABLE 2b TO PART 679—SPECIES CODES: FMP PROHIBITED SPECIES AND CR CRAB Species Description Code CR Crab Groundfish PSC CRAB Box ....................................... Lopholithodes mandtii .......... 900 ✓ Dungeness ........................... Cancer magister .................. 910 ✓ King, blue ............................. Paralithodes platypus .......... 922 ✓ ✓ King, golden (brown) ........... Lithodes aequispinus ........... 923 ✓ ✓ King, red .............................. Paralithodes camtshaticus ... 921 ✓ ✓ King, scarlet (deepsea) ....... Lithodes couesi .................... 924 ✓ Korean horsehair crab ......... Erimacrus isenbeckii ............ 940 ✓ Multispinus crab ................... Paralomis multispinus .......... 951 ✓ Tanner, Bairdi ...................... Chionoecetes bairdi ............. 931 ✓ ✓ Tanner, grooved .................. Chionoecetes tanneri ........... 933 ✓ Tanner, snow ....................... Chionoecetes opilio ............. 932 ✓ ✓ Tanner, triangle ................... Chionoecetes angulatus ...... 934 ✓ Verrilli crab ........................... Paralomis verrilli .................. 953 ✓ PACIFIC HALIBUT Hippoglossus stenolepis ...... 200 ✓ PACIFIC HERRING Family Clupeidae ................. 235 ✓ SALMON Chinook (king) ..................... Oncorhynchus tshawytscha 410 ✓ Chum (dog) .......................... Oncorhynchus keta .............. 450 ✓ Coho (silver) ........................ Oncorhynchus kisutch ......... 430 ✓ Pink (humpback) ................. -

655 Appendix G
APPENDIX G: GLOSSARY Appendix G-1. Demersal Fish Species Alphabetized by Species Name. ....................................... G1-1 Appendix G-2. Demersal Fish Species Alphabetized by Common Name.. .................................... G2-1 Appendix G-3. Invertebrate Species Alphabetized by Species Name.. .......................................... G3-1 Appendix G-4. Invertebrate Species Alphabetized by Common Name.. ........................................ G4-1 G-1 Appendix G-1. Demersal Fish Species Alphabetized by Species Name. Demersal fish species collected at depths of 2-484 m on the southern California shelf and upper slope, July-October 2008. Species Common Name Agonopsis sterletus southern spearnose poacher Anchoa compressa deepbody anchovy Anchoa delicatissima slough anchovy Anoplopoma fimbria sablefish Argyropelecus affinis slender hatchetfish Argyropelecus lychnus silver hachetfish Argyropelecus sladeni lowcrest hatchetfish Artedius notospilotus bonyhead sculpin Bathyagonus pentacanthus bigeye poacher Bathyraja interrupta sandpaper skate Careproctus melanurus blacktail snailfish Ceratoscopelus townsendi dogtooth lampfish Cheilotrema saturnum black croaker Chilara taylori spotted cusk-eel Chitonotus pugetensis roughback sculpin Citharichthys fragilis Gulf sanddab Citharichthys sordidus Pacific sanddab Citharichthys stigmaeus speckled sanddab Citharichthys xanthostigma longfin sanddab Cymatogaster aggregata shiner perch Embiotoca jacksoni black perch Engraulis mordax northern anchovy Enophrys taurina bull sculpin Eopsetta jordani