Vascular and Extravascular Fluid Volumes In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stomach Content Analysis of Short-Finned Pilot Whales
f MARCH 1986 STOMACH CONTENT ANALYSIS OF SHORT-FINNED PILOT WHALES h (Globicephala macrorhynchus) AND NORTHERN ELEPHANT SEALS (Mirounga angustirostris) FROM THE SOUTHERN CALIFORNIA BIGHT by Elizabeth S. Hacker ADMINISTRATIVE REPORT LJ-86-08C f This Administrative Report is issued as an informal document to ensure prompt dissemination of preliminary results, interim reports and special studies. We recommend that it not be abstracted or cited. STOMACH CONTENT ANALYSIS OF SHORT-FINNED PILOT WHALES (GLOBICEPHALA MACRORHYNCHUS) AND NORTHERN ELEPHANT SEALS (MIROUNGA ANGUSTIROSTRIS) FROM THE SOUTHERN CALIFORNIA BIGHT Elizabeth S. Hacker College of Oceanography Oregon State University Corvallis, Oregon 97331 March 1986 S H i I , LIBRARY >66 MAR 0 2 2007 ‘ National uooarac & Atmospheric Administration U.S. Dept, of Commerce This report was prepared by Elizabeth S. Hacker under contract No. 84-ABA-02592 for the National Marine Fisheries Service, Southwest Fisheries Center, La Jolla, California. The statements, findings, conclusions and recommendations herein are those of the author and do not necessarily reflect the views of the National Marine Fisheries Service. Charles W. Oliver of the Southwest Fisheries Center served as Contract Officer's Technical Representative for this contract. ADMINISTRATIVE REPORT LJ-86-08C CONTENTS PAGE INTRODUCTION.................. 1 METHODS....................... 2 Sample Collection........ 2 Sample Identification.... 2 Sample Analysis.......... 3 RESULTS....................... 3 Globicephala macrorhynchus 3 Mirounga angustirostris... 4 DISCUSSION.................... 6 ACKNOWLEDGEMENTS.............. 11 REFERENCES.............. 12 i LIST OF TABLES TABLE PAGE 1 Collection data for Globicephala macrorhynchus examined from the Southern California Bight........ 19 2 Collection data for Mirounga angustirostris examined from the Southern California Bight........ 20 3 Stomach contents of Globicephala macrorhynchus examined from the Southern California Bight....... -

Lamprey, Hagfish
Agnatha - Lamprey, Kingdom: Animalia Phylum: Chordata Super Class: Agnatha Hagfish Agnatha are jawless fish. Lampreys and hagfish are in this class. Members of the agnatha class are probably the earliest vertebrates. Scientists have found fossils of agnathan species from the late Cambrian Period that occurred 500 million years ago. Members of this class of fish don't have paired fins or a stomach. Adults and larvae have a notochord. A notochord is a flexible rod-like cord of cells that provides the main support for the body of an organism during its embryonic stage. A notochord is found in all chordates. Most agnathans have a skeleton made of cartilage and seven or more paired gill pockets. They have a light sensitive pineal eye. A pineal eye is a third eye in front of the pineal gland. Fertilization of eggs takes place outside the body. The lamprey looks like an eel, but it has a jawless sucking mouth that it attaches to a fish. It is a parasite and sucks tissue and fluids out of the fish it is attached to. The lamprey's mouth has a ring of cartilage that supports it and rows of horny teeth that it uses to latch on to a fish. Lampreys are found in temperate rivers and coastal seas and can range in size from 5 to 40 inches. Lampreys begin their lives as freshwater larvae. In the larval stage, lamprey usually are found on muddy river and lake bottoms where they filter feed on microorganisms. The larval stage can last as long as seven years! At the end of the larval state, the lamprey changes into an eel- like creature that swims and usually attaches itself to a fish. -

Reporting for the Period from May 2015-April 2016
Washington Contribution to the 2016 Meeting of the Technical Sub-Committee (TSC) of the Canada-U.S. Groundfish Committee: Reporting for the period from May 2015-April 2016 April 26th-27th, 2016 Edited by: Dayv Lowry Contributions by: Dayv Lowry Robert Pacunski Lorna Wargo Mike Burger Taylor Frierson Todd Sandell Jen Blaine Brad Speidel Larry LeClair Phil Weyland Donna Downs Theresa Tsou Washington Department of Fish and Wildlife April 2016 Contents I. Agency Overview.....................................................................................................................3 II. Surveys.....................................................................................................................................4 III. Reserves..............................................................................................................................16 IV. Review of Agency Groundfish Research, Assessment, and Management.........................16 A. Hagfish............................................................................................................................16 B. North Pacific Spiny Dogfish and other sharks................................................................20 C. Skates..............................................................................................................................20 D. Pacific Cod......................................................................................................................20 E. Walleye Pollock..............................................................................................................21 -

Eptatretus Stoutii)
CARDIAC CONTROL IN THE PACIFIC HAGFISH (EPTATRETUS STOUTII) by Christopher Mark Wilson B.Sc., University of Manchester, 2007 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in The Faculty of Graduate and Postdoctoral Studies (Zoology) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) October 2014 © Christopher Mark Wilson, 2014 ABSTRACT The Pacific hagfish (Eptatretus stoutii), being an extant ancestral craniate, possesses the most ancestral craniate-type heart with valved chambers, a response to increased filling pressure with increased stroke volume (Frank-Starling mechanism), and myogenic contractions. Unlike all other known craniate hearts, this heart receives no direct neural stimulation. Despite this, heart rate can vary four-fold during a prolonged, 36-h anoxic challenge followed by a normoxic recovery period, with heart rate decreasing in anoxia, and increasing beyond routine rates during recovery, a remarkable feat for an aneural heart. This thesis is a study of how the hagfish can regulate heart rate without the assistance of neural stimulation. A major role of hyperpolarization-activated cyclic nucleotide-activated (HCN) channels in heartbeat initiation was indicated by pharmacological application of zatebradine to spontaneously contracting, isolated hearts, which stopped atrial contraction and vastly reduced ventricular contraction. Tetrodotoxin inhibition of voltage-gated Na+ channels induced an atrioventricular block suggesting these channels play a role in cardiac conduction. Partial cloning of HCN channel mRNA extracted from hagfish hearts revealed six HCN isoforms, two hagfish representatives of vertebrate HCN2 (HCN2a and HCN2b), three of HCN3 (HCN3a, HCN3b and HCN3c) and one HCN4. Two paralogs of HCN3b were discovered, however, HCN3a dominated the expression of ii HCN isoforms followed by HCN4. -

Evaluation of O2 Uptake in Pacific Hagfish Refutes a Major Respiratory Role for the Skin Alexander M
© 2016. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2016) 219, 2814-2818 doi:10.1242/jeb.141598 SHORT COMMUNICATION It’s all in the gills: evaluation of O2 uptake in Pacific hagfish refutes a major respiratory role for the skin Alexander M. Clifford1,2,*, Alex M. Zimmer2,3, Chris M. Wood2,3 and Greg G. Goss1,2 ABSTRACT hagfishes, citing the impracticality for O2 exchange across the – Hagfish skin has been reported as an important site for ammonia 70 100 µm epidermal layer, perfusion of capillaries with arterial excretion and as the major site of systemic oxygen acquisition. blood of high PO2 and the impact of skin boundary layers on diffusion. However, whether cutaneous O2 uptake is the dominant route of uptake remains under debate; all evidence supporting this Here, we used custom-designed respirometry chambers to isolate hypothesis has been derived using indirect measurements. Here, anterior (branchial+cutaneous) and posterior (cutaneous) regions of we used partitioned chambers and direct measurements of oxygen Pacific hagfish [Eptatretus stoutii (Lockington 1878)] to partition whole-animal Ṁ and ammonia excretion (J ). Exercise consumption and ammonia excretion to quantify cutaneous and O2 ̇ Amm branchial exchanges in Pacific hagfish (Eptatretus stoutii) at rest and typically leads to increases in MO2 and JAmm during post-exercise following exhaustive exercise. Hagfish primarily relied on the gills for recovery; therefore, we employed exhaustive exercise to determine the relative contribution of the gills and skin to elevations in both O2 uptake (81.0%) and ammonia excretion (70.7%). Following metabolic demand. Given that skin is proposed as the primary site exercise, both O2 uptake and ammonia excretion increased, but only ̇ across the gill; cutaneous exchange was not increased. -

Jawless Fishes of the World
Jawless Fishes of the World Jawless Fishes of the World: Volume 1 Edited by Alexei Orlov and Richard Beamish Jawless Fishes of the World: Volume 1 Edited by Alexei Orlov and Richard Beamish This book first published 2016 Cambridge Scholars Publishing Lady Stephenson Library, Newcastle upon Tyne, NE6 2PA, UK British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Copyright © 2016 by Alexei Orlov, Richard Beamish and contributors All rights for this book reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner. ISBN (10): 1-4438-8582-7 ISBN (13): 978-1-4438-8582-9 TABLE OF CONTENTS Volume 1 Preface ........................................................................................................ ix M. Docker Part 1: Evolution, Phylogeny, Diversity, and Taxonomy Chapter One ................................................................................................. 2 Molecular Evolution in the Lamprey Genomes and Its Relevance to the Timing of Whole Genome Duplications T. Manousaki, H. Qiu, M. Noro, F. Hildebrand, A. Meyer and S. Kuraku Chapter Two .............................................................................................. 17 Molecular Phylogeny and Speciation of East Asian Lampreys (genus Lethenteron) with reference to their Life-History Diversification Y. Yamazaki and -

THE PINNIPEDS of the CALIFORNIA CURRENT California
ANTONELIS AND FISCUS: PINNIPEDS OF THE CALIFORNIA CURRENT CalCOFI Rep., Vol. XXI, 1980 THE PINNIPEDS OF THE CALIFORNIA CURRENT GEORGE A. ANTONELIS. JR. AND CLIFFORD H. FISCUS Marine Mammal Division Northwest and Alaska Fisheries Center National Marine Fisheries Service National Oceanic and Atmospheric Administration 7600 Sand Point Way, N.E. Seattle, WA 981 15 ABSTRACT 10s pequenos peces en 10s cardumenes y peces ana- There are six species of pinnipeds-California sea dromos. Los dos focidos, otra vez con ciertas excep- lion, Zalophus californianus; northern sea lion, Eume- ciones, predan especies diferentes. Aparentemente, el topias jubatus; northern fur seal, Callorhinus ursinus; elefante marino se alimenta en aguas mas profundas que Guadalupe fur seal, Arctocephalus townsendi; harbor la foca peluda, alimentindose de especies demersales seal, Phoca uitulina richardsi; and northern elephant y benticas, y la foca peluda se alimenta de especiesdemer- seal, Mirounga angustirostris-that inhabit the study sales costeras y neriticas, entrando ocasionalmente en rios area of the California Cooperative Oceanic Fisheries y aguas estuarinas haciendopresa de 10s peces anadromos Investigations (CalCOFI). y otros pequeiios peces que entran regularmente en estas The numbers of animals in each population are given; aguas. the size, distribution, and seasonal movements are de- scribed. The known prey species of the pinnipeds are INTRODUCTION listed for each species. The otariids, with certain excep- The California Current, its components, and the Cali- tions, consume the same kinds of prey, although in slight- fornia Cooperative Oceanic Fisheries Investigations ly different amounts. In general they feed most commonly (CalCOFI) station plan have been described many times on the smaller schooling fishes and squids of the epi- in the past and are well known (Kramer et al. -

Characterizing the Deep: Surveys in the Monterey Bay National Marine Sanctuary 2007-2010 Acknowledgments
Characterizing the Deep: Surveys in the Monterey Bay National Marine Sanctuary 2007-2010 Acknowledgments enerous support for this project comes from the Monterey Bay Many thanks also to the Seafloor Mapping Lab at CSUMB, the US GNational Marine Sanctuary as well as California State University Geological Survey, and Monterey Bay Aquarium Research Institute for Monterey Bay’s Undergraduate Research Opportunities Center and the topographic maps of the seafloor throughout the study areas. James W. Rote Distinguished Professorship in Marine Science and Policy. All photos in this document (unless otherwise noted) were taken with This publication was conceived and implemented by the faculty, the ROV Beagle in the Monterey Bay National Marine Sanctuary. Credit staff and students of the Institute for Applied Marine Ecology at is to: IfAME/CSUMB/MBNMS/NOAA/MARE CSU Monterey Bay… Topside operations photos credit to Adam Alfasso, Ashley Knight, Heather Dr. James Lindholm – Founder & Director Kramp, Andrew DeVogelaere, Jason Adelaars Ashley Knight – Research Technician Jason Adelaars – Graduate Research Intern Special thanks to Robert Lea, Jean de Marignac, Donna Kline, and Bryon Megan Kelly – Graduate Research Assistant Downey for help with species identifications. Also many thanks to Heather Kramp – Undergraduate Research Assistant Judy Anderson (A Graphic Design Studio) for her expertise, talent, and patience with us in designing this report. ...with key support from Dr. Andrew DeVogelaere, Research Coordinator for the Monterey Bay National Marine -

The Biology of Hagfishes the Biology of Hagfishes
THE BIOLOGY OF HAGFISHES THE BIOLOGY OF HAGFISHES J0RGEN M0RUP J0RGENSEN, JENS PETER LOMHOLT, ROY E. WEBER and HANSMALTE Department of Zoophysiology Institute of Biological Sciences University of Aarhus Denmark luni Springer-Science+Business Media, B.V. Published by Chapman and HaU, an imprint of Thomson Science, 2--6 Boundary Row, London SEl 8HN, UK Thomson Science, 2-6 Boundary Row, London SEl 8HN, UK Thomson Science, 115 Fifth Avenue, New York, NY 10003, USA Thomson Science, Suite 750, 400 Market Street, Philadelphia, PA 19106, USA Thomson Science, Pappelallee 3, 694469 Weinheim, Germany First edition 1998 © 1998 Springer Science+ Business Media Dordrecht OriginaUy published by Chapman & HaU Ltdin 1998 Softcover reprint of the hardcover 1st edition 1998 Typeset in 10112pt Palatino by Cambrian Typesetters, Frimley, Surrey ISBN 978-94-010-6465-1 ISBN 978-94-011-5834-3 (eBook) DOI 10.1007/978-94-011-5834-3 AII rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, e1e~tronic, mechanical, photocopying, recording or otherwise, without the prior written permis sion of the publishers. Applications for permission shou1d be addressed to the rights manager at the London address of the publisher. The publisher makes no representation, express or imp1ied, with regard to the accuracy of the information contained in this book and cannot accept any legal responsibility or liability for any errors or omissions that may be made. A catalogue record for this book is available from the British Library Library ofCongress Catalog Card Number: 97-69524 8 Printed on acid-free text paper, manufactured in accordance with ANSIINISO Z39.48-1992 (Permanence of Paper). -
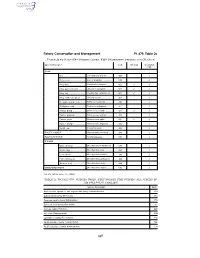
Species Codes: Fmp Prohibited Species and Cr Crab
Fishery Conservation and Management Pt. 679, Table 2c TABLE 2b TO PART 679—SPECIES CODES: FMP PROHIBITED SPECIES AND CR CRAB Species Description Code CR Crab Groundfish PSC CRAB Box ....................................... Lopholithodes mandtii .......... 900 ✓ Dungeness ........................... Cancer magister .................. 910 ✓ King, blue ............................. Paralithodes platypus .......... 922 ✓ ✓ King, golden (brown) ........... Lithodes aequispinus ........... 923 ✓ ✓ King, red .............................. Paralithodes camtshaticus ... 921 ✓ ✓ King, scarlet (deepsea) ....... Lithodes couesi .................... 924 ✓ Korean horsehair crab ......... Erimacrus isenbeckii ............ 940 ✓ Multispinus crab ................... Paralomis multispinus .......... 951 ✓ Tanner, Bairdi ...................... Chionoecetes bairdi ............. 931 ✓ ✓ Tanner, grooved .................. Chionoecetes tanneri ........... 933 ✓ Tanner, snow ....................... Chionoecetes opilio ............. 932 ✓ ✓ Tanner, triangle ................... Chionoecetes angulatus ...... 934 ✓ Verrilli crab ........................... Paralomis verrilli .................. 953 ✓ PACIFIC HALIBUT Hippoglossus stenolepis ...... 200 ✓ PACIFIC HERRING Family Clupeidae ................. 235 ✓ SALMON Chinook (king) ..................... Oncorhynchus tshawytscha 410 ✓ Chum (dog) .......................... Oncorhynchus keta .............. 450 ✓ Coho (silver) ........................ Oncorhynchus kisutch ......... 430 ✓ Pink (humpback) ................. -

655 Appendix G
APPENDIX G: GLOSSARY Appendix G-1. Demersal Fish Species Alphabetized by Species Name. ....................................... G1-1 Appendix G-2. Demersal Fish Species Alphabetized by Common Name.. .................................... G2-1 Appendix G-3. Invertebrate Species Alphabetized by Species Name.. .......................................... G3-1 Appendix G-4. Invertebrate Species Alphabetized by Common Name.. ........................................ G4-1 G-1 Appendix G-1. Demersal Fish Species Alphabetized by Species Name. Demersal fish species collected at depths of 2-484 m on the southern California shelf and upper slope, July-October 2008. Species Common Name Agonopsis sterletus southern spearnose poacher Anchoa compressa deepbody anchovy Anchoa delicatissima slough anchovy Anoplopoma fimbria sablefish Argyropelecus affinis slender hatchetfish Argyropelecus lychnus silver hachetfish Argyropelecus sladeni lowcrest hatchetfish Artedius notospilotus bonyhead sculpin Bathyagonus pentacanthus bigeye poacher Bathyraja interrupta sandpaper skate Careproctus melanurus blacktail snailfish Ceratoscopelus townsendi dogtooth lampfish Cheilotrema saturnum black croaker Chilara taylori spotted cusk-eel Chitonotus pugetensis roughback sculpin Citharichthys fragilis Gulf sanddab Citharichthys sordidus Pacific sanddab Citharichthys stigmaeus speckled sanddab Citharichthys xanthostigma longfin sanddab Cymatogaster aggregata shiner perch Embiotoca jacksoni black perch Engraulis mordax northern anchovy Enophrys taurina bull sculpin Eopsetta jordani -

Deepwater Demersal Fishes Observed from the Submersible 24 June 1985
Deepwater demersal fishes observed from the submersible Avalon (DSRV-2) off the Farallon Islands, 24 June 1985 Item Type monograph Authors Gotshall, Daniel W.; Dyer, Robert S. Publisher California Department of Fish and Game Download date 29/09/2021 03:56:50 Link to Item http://hdl.handle.net/1834/18624 DEEPWATER DEMERSAL FISHES OBSERVED FROM THE SUBMERSIBLE AVALON (DSRV-2~ OFF THE FARALLON ISLANDS, 24 JUNE 1985 by DANIEL W. GOTSHALL and ROBERT S. DYER MARINE RESOURCES TECHNICAL REPORT NO. 55 1987 CALIFORNIA DEPARTMENT OF FISH AND GAME MARINE RESOURCES TECHNICAL REPORTS ************************************** Marine Resources Technical Reports are research documents by Department personnel that are of sufficient importance to be preserved, but which for some reason are not appropriate for primary scientific publication. No restriction is placed on subject matter. These Reports may be cited in publication. The material in these Reports may eventually appear in the primary scientific literature. Inquiries concerning the Reports should be directed to The Editor, Marine Resources Division, Department of Fish and Game, 1301 West 12th St., Long Beach, California 90813. DEEPWATER DEMERSAL FISHES OBSERVED FROM THE SUBMERSIBLE AVALON (DSRV-2) OFF THE FARALLON ISLANDS, 24 JUNE 1985 by Daniel W. Gotshall Marine Resources Division California Department of Fish and Game and Robert S. Dyer Office of Radiation Programs U.S. Environmental Protection Agency MARINE RESOURCES TECHNICAL REPORT NO. 55 California Department of Fish and Game 1987 ABSTRACT On 24 June 1985 the u.S. Navy's Deep Submergence Rescue Vehicle AVALON [DSRV-2] was used to locate low-level radioactive waste containers and make observations of deepwater benthic fishes and invertebrates at the 900 m (2952 ft) radioactive waste disposal site approximat1y 4.4 km SW of the Fara110n Islands off San Francisco, California.