Significant Association Between Butyric Acid and Celiac Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Propionic Acid on Fatty Acid Oxidation and U Reagenesis
Pediat. Res. 10: 683- 686 (1976) Fatty degeneration propionic acid hyperammonemia propionic acidemia liver ureagenesls Effect of Propionic Acid on Fatty Acid Oxidation and U reagenesis ALLEN M. GLASGOW(23) AND H. PET ER C HASE UniversilY of Colorado Medical Celller, B. F. SlOlillsky LaboralOries , Denver, Colorado, USA Extract phosphate-buffered salin e, harvested with a brief treatment wi th tryps in- EDTA, washed twice with ph os ph ate-buffered saline, and Propionic acid significantly inhibited "CO z production from then suspended in ph os ph ate-buffe red saline (145 m M N a, 4.15 [I-"ejpalmitate at a concentration of 10 11 M in control fibroblasts m M K, 140 m M c/, 9.36 m M PO" pH 7.4) . I n mos t cases the cells and 100 11M in methyl malonic fibroblasts. This inhibition was we re incubated in 3 ml phosph ate-bu ffered sa lin e cont aining 0.5 similar to that produced by 4-pentenoic acid. Methylmalonic acid I1Ci ll-I4Cj palm it ate (19), final concentration approximately 3 11M also inhibited ' 'C0 2 production from [V 'ejpalmitate, but only at a added in 10 II I hexane. Increasing the amount of hexane to 100 II I concentration of I mM in control cells and 5 mM in methyl malonic did not impair palmit ate ox id ation. In two experiments (Fig. 3) the cells. fibroblasts were in cub ated in 3 ml calcium-free Krebs-Ringer Propionic acid (5 mM) also inhibited ureagenesis in rat liver phosphate buffer (2) co nt ain in g 5 g/ 100 ml essent iall y fatty ac id slices when ammonia was the substrate but not with aspartate and free bovine se rum albumin (20), I mM pa lm itate, and the same citrulline as substrates. -

Chapter 21 the Chemistry of Carboxylic Acid Derivatives
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 21 The Chemistry of Carboxylic Acid Derivatives Solutions to In-Text Problems 21.1 (b) (d) (e) (h) 21.2 (a) butanenitrile (common: butyronitrile) (c) isopentyl 3-methylbutanoate (common: isoamyl isovalerate) The isoamyl group is the same as an isopentyl or 3-methylbutyl group: (d) N,N-dimethylbenzamide 21.3 The E and Z conformations of N-acetylproline: 21.5 As shown by the data above the problem, a carboxylic acid has a higher boiling point than an ester because it can both donate and accept hydrogen bonds within its liquid state; hydrogen bonding does not occur in the ester. Consequently, pentanoic acid (valeric acid) has a higher boiling point than methyl butanoate. Here are the actual data: INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 21 2 21.7 (a) The carbonyl absorption of the ester occurs at higher frequency, and only the carboxylic acid has the characteristic strong, broad O—H stretching absorption in 2400–3600 cm–1 region. (d) In N-methylpropanamide, the N-methyl group is a doublet at about d 3. N-Ethylacetamide has no doublet resonances. In N-methylpropanamide, the a-protons are a quartet near d 2.5. In N-ethylacetamide, the a- protons are a singlet at d 2. The NMR spectrum of N-methylpropanamide has no singlets. 21.9 (a) The first ester is more basic because its conjugate acid is stabilized not only by resonance interaction with the ester oxygen, but also by resonance interaction with the double bond; that is, the conjugate acid of the first ester has one more important resonance structure than the conjugate acid of the second. -

Thermal Stability of Oleic Acid and Ethyl Linoleate
Chapter 3.1 ____________________________________________________________________ Thermal Stability of Oleic Acid and Ethyl Linoleate The first part of this work consisted of studying the thermal stability of oleic acid, which was initially a candidate as a starting material for the dehydrogenation of fatty acids using heterogeneous catalysis. The results induced us to search another starting material and to study its properties. 3.1.1 Introduction Oil-derived products can be subjected to conditions that promote oxidation of their unsaturated components during storage and use. The materials arising during oxidation and subsequent degradation can seriously impair the quality and performance of such products [1, 2]. Thus, oxidative stability is an important issue to face before studying their catalytic reactivity. The fatty acid alkyl chain is susceptible to oxidation both at double bonds and adjacent allylic carbons. Free-radical and photooxidation at allylic carbons are responsible for deterioration of unsaturated oils and fats [2-6]. Both autoxidation and photooxidation produce hydroperoxides in allylic bonds. During this process, the position and geometry of the double bond may change. The hydroperoxide mixtures produced by autoxidation and photooxidation are not identical, indicating that different mechanisms are involved [4-6]. The autoxidation reaction occurs in the presence of oxygen. Autoxidation is a free-radical chain reaction, which involves the series of reactions that initiate, propagate and terminate the chain [3]. • initiation RH → R • + → • propagation R O2 ROO fast • • ROO + RH → ROOH + R rate determining • • termination R , ROO → stable products The initiator is a free radical, most probably produced by decomposition of hydroperoxides already present or produced by photooxidation. -

The Role of Short Chain Fatty Acids in Appetite Regulation and Energy Homeostasis
OPEN International Journal of Obesity (2015) 39, 1331–1338 © 2015 Macmillan Publishers Limited All rights reserved 0307-0565/15 www.nature.com/ijo REVIEW The role of short chain fatty acids in appetite regulation and energy homeostasis CS Byrne1, ES Chambers1, DJ Morrison2 and G Frost1 Over the last 20 years there has been an increasing interest in the influence of the gastrointestinal tract on appetite regulation. Much of the focus has been on the neuronal and hormonal relationship between the gastrointestinal tract and the brain. There is now mounting evidence that the colonic microbiota and their metabolic activity have a significant role in energy homeostasis. The supply of substrate to the colonic microbiota has a major impact on the microbial population and the metabolites they produce, particularly short chain fatty acids (SCFAs). SCFAs are produced when non-digestible carbohydrates, namely dietary fibres and resistant starch, undergo fermentation by the colonic microbiota. Both the consumption of fermentable carbohydrates and the administration of SCFAs have been reported to result in a wide range of health benefits including improvements in body composition, glucose homeostasis, blood lipid profiles and reduced body weight and colon cancer risk. However, published studies tend to report the effects that fermentable carbohydrates and SCFAs have on specific tissues and metabolic processes, and fail to explain how these local effects translate into systemic effects and the mitigation of disease risk. Moreover, studies tend to investigate SCFAs collectively and neglect to report the effects associated with individual SCFAs. Here, we bring together the recent evidence and suggest an overarching model for the effects of SCFAs on one of their beneficial aspects: appetite regulation and energy homeostasis. -

Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis
International Journal of Molecular Sciences Review Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis David Johane Machate 1, Priscila Silva Figueiredo 2 , Gabriela Marcelino 2 , Rita de Cássia Avellaneda Guimarães 2,*, Priscila Aiko Hiane 2 , Danielle Bogo 2, Verônica Assalin Zorgetto Pinheiro 2, Lincoln Carlos Silva de Oliveira 3 and Arnildo Pott 1 1 Graduate Program in Biotechnology and Biodiversity in the Central-West Region of Brazil, Federal University of Mato Grosso do Sul, Campo Grande 79079-900, Brazil; [email protected] (D.J.M.); [email protected] (A.P.) 2 Graduate Program in Health and Development in the Central-West Region of Brazil, Federal University of Mato Grosso do Sul, Campo Grande 79079-900, Brazil; pri.fi[email protected] (P.S.F.); [email protected] (G.M.); [email protected] (P.A.H.); [email protected] (D.B.); [email protected] (V.A.Z.P.) 3 Chemistry Institute, Federal University of Mato Grosso do Sul, Campo Grande 79079-900, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-67-3345-7416 Received: 9 March 2020; Accepted: 27 March 2020; Published: 8 June 2020 Abstract: Long-term high-fat dietary intake plays a crucial role in the composition of gut microbiota in animal models and human subjects, which affect directly short-chain fatty acid (SCFA) production and host health. This review aims to highlight the interplay of fatty acid (FA) intake and gut microbiota composition and its interaction with hosts in health promotion and obesity prevention and its related metabolic dysbiosis. -
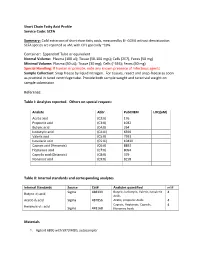
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -

Fatty Acids: Essential…Therapeutic
Volume 3, No.2 May/June 2000 A CONCISE UPDATE OF IMPORTANT ISSUES CONCERNING NATURAL HEALTH INGREDIENTS Written and Edited By: Thomas G. Guilliams Ph.D. FATTY ACIDS: Essential...Therapeutic Few things have been as confusing to both patient and health care provider as the issue of fats and oils. Of all the essential nutrients required for optimal health, fatty acids have not only been forgotten they have been considered hazardous. Health has somehow been equated with “low-fat” or “fat-free” for so long, to suggest that fats could be essential or even therapeutic is to risk credibility. We hope to give a view of fats that is both balanced and scientific. This review will cover the basics of most fats that will be encountered in dietary or supplemental protocols. Recommendations to view essential fatty acids in a similar fashion as essential vitamins and minerals will be combined with therapeutic protocols for conditions ranging from cardiovascular disease, skin conditions, diabetes, nerve related disorders, retinal disorders and more. A complete restoration of health cannot be accomplished until there is a restoration of fatty acid nutritional information among health care professionals and their patients. Fats- What are they? Dietary fats come to us from a variety of sources, but primarily in the form of triglycerides. That is, three fatty acid molecules connected by a glycerol backbone (see fatty acid primer page 3 for diagram). These fatty acids are then used as energy by our cells or modified into phospholipids to be used as cell or organelle membranes. Some fatty acids are used in lipoprotein molecules to shuttle cholesterol and fats to and from cells, and fats may also be stored for later use. -

Valproic Acid and Its Amidic Derivatives As New Antivirals Against Alphaherpesviruses
viruses Review Valproic Acid and Its Amidic Derivatives as New Antivirals against Alphaherpesviruses Sabina Andreu 1,2,* , Inés Ripa 1,2, Raquel Bello-Morales 1,2 and José Antonio López-Guerrero 1,2 1 Departamento de Biología Molecular, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain; [email protected] (I.R.); [email protected] (R.B.-M.); [email protected] (J.A.L.-G.) 2 Centro de Biología Molecular Severo Ochoa, Spanish National Research Council—Universidad Autónoma de Madrid (CSIC-UAM), Cantoblanco, 28049 Madrid, Spain * Correspondence: [email protected] Academic Editor: Maria Kalamvoki Received: 14 November 2020; Accepted: 25 November 2020; Published: 26 November 2020 Abstract: Herpes simplex viruses (HSVs) are neurotropic viruses with broad host range whose infections cause considerable health problems in both animals and humans. In fact, 67% of the global population under the age of 50 are infected with HSV-1 and 13% have clinically recurrent HSV-2 infections. The most prescribed antiherpetics are nucleoside analogues such as acyclovir, but the emergence of mutants resistant to these drugs and the lack of available vaccines against human HSVs has led to an imminent need for new antivirals. Valproic acid (VPA) is a branched short-chain fatty acid clinically used as a broad-spectrum antiepileptic drug in the treatment of neurological disorders, which has shown promising antiviral activity against some herpesviruses. Moreover, its amidic derivatives valpromide and valnoctamide also share this antiherpetic activity. This review summarizes the current research on the use of VPA and its amidic derivatives as alternatives to traditional antiherpetics in the fight against HSV infections. -
![[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An](https://docslib.b-cdn.net/cover/9902/agr-biol-chem-vol-36-no-1-p-112-119-1972-studies-of-the-peptide-antibiotic-suzukacillin-part-ii-by-takaaki-ooka-an-399902.webp)
[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An
[Agr. Biol. Chem., Vol. 36, No. 1, p. 112---119,1972] Studies of the Peptide Antibiotic Suzukacillin Part II By Takaaki OOKA and Isao TAKEDA TechnicalResearch Laboratory, Asahi ChemicalIndustry Co., Ltd., Nakadai-cho3-27, Itabashi-kuTokyo ReceivedJuly 26, 1971 Suzukacillin produced by Trichoderma viride 6301 strain was purified and shown to be separated into two components A and B on thin-layer chromatography. The component A was isolated and crystallized from the mixture of components by alumina column chro matography. The component A is composed of six amino acids, Gly, Glu, Ala, Pro, Val, Leu and an unknown amino acid. This unknown amino acid was identified as a-amino isobutyric acid. It is supposed that a-amino isobutyrie acid is biosynthesized mainly from L-valine by the isotopic experiments. Suzukacillin formation by Trichoderma viride 6301 was stimulated by the addition of L-Asn, GABA, L-Ser, Gly and L-Arg into the medium. Trichoderma viride 6301 strain producing Suzu ments were purchased as reagents from Tokyo Kasei kacillin was isolated by the author's laboratory. Co., Ltd. The following radioactive compounds were This organism accumulated noted amount of surplied by Dai-ichi Chemical Co., Ltd., with the in the peptide antibiotic. It was detected on dicated specific activities (ƒÊCi per mmole): Starch- TLC that the peptide antibiotic Suzukacillin (U)14C (30.8), pyruvic acid-(U)-14C (31.5), glycine- consists of two components A and B. A is (U)14C (45.2), L-alanine-(U)-14C (107), L-glutamic acid- (U)-14C (165), L-proline-(U)-14C (165), L-valine-(U)-14C a major and B is a minor component. -

The Lysis of Bacterium Coli by Amino-Acids
[510 ] THE LYSIS OF BACTERIUM COLI BY AMINO-ACIDS BY J. GORDON, R. A. HALL AND L. H. STICKLAND The School of Medicine, Leeds (With 2 Figures in the Text) Gordon, Hall & Stickland (1951, 1953) described the kinetics of the lysis by glycine of Bacterium coli suspensions, and the inhibition by various agents of this process. The present paper attempts to correlate this lytic effect with the chemical structure of various amino-acids. MATERIALS AND METHODS Preparation of bacteria Four freshly isolated strains of Bact. coli were grown on the surface of nutrient agar for 16-18 hr. at 37°, and were then washed off with distilled water. These suspensions were filtered through glass wool. The bacteria were washed twice and finally suspended in about ten times their volume of distilled water which was equivalent to a concentration of between 10 and 20 mg. dry weight of cells per ml. Measurement of lysis After the suspensions had been incubated with the amino-acid solutions under test the bacteria were removed by centrifuging at 2500 g. for 20 min. The super- natant fluid was treated with \ volume of 25 % ' w/v' trichloracetic acid solution, the protein precipitate was washed with 5% trichloracetic acid and then the protein was estimated by the biuret method (Robinson & Hogden, 1940). The degree of lysis was expressed as the ratio of the protein liberated into the super- natant fluid to the total soluble protein of the bacterial suspension (Stickland, 1951). Amino-acids used Glycine, DL-alanine, /?-alanine, DL-a-amino-w-butyric acid, DL-a-amino-iso- butyric acid, DL-a-amino-?i-valeric acid, DL-valine, DL-serine, glycine ethyl ester hydrochloride, aceturic acid, glycylglycine and L-glutamic acid were obtained commercially. -

Fatty Acid Biosynthesis
BI/CH 422/622 ANABOLISM OUTLINE: Photosynthesis Carbon Assimilation – Calvin Cycle Carbohydrate Biosynthesis in Animals Gluconeogenesis Glycogen Synthesis Pentose-Phosphate Pathway Regulation of Carbohydrate Metabolism Anaplerotic reactions Biosynthesis of Fatty Acids and Lipids Fatty Acids contrasts Diversification of fatty acids location & transport Eicosanoids Synthesis Prostaglandins and Thromboxane acetyl-CoA carboxylase Triacylglycerides fatty acid synthase ACP priming Membrane lipids 4 steps Glycerophospholipids Control of fatty acid metabolism Sphingolipids Isoprene lipids: Cholesterol ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1 ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1. Biosynthesis of fatty acids 2. Regulation of fatty acid degradation and synthesis 3. Assembly of fatty acids into triacylglycerol and phospholipids 4. Metabolism of isoprenes a. Ketone bodies and Isoprene biosynthesis b. Isoprene polymerization i. Cholesterol ii. Steroids & other molecules iii. Regulation iv. Role of cholesterol in human disease ANABOLISM II: Biosynthesis of Fatty Acids & Lipids Lipid Fat Biosynthesis Catabolism Fatty Acid Fatty Acid Degradation Synthesis Ketone body Isoprene Utilization Biosynthesis 2 Catabolism Fatty Acid Biosynthesis Anabolism • Contrast with Sugars – Lipids have have hydro-carbons not carbo-hydrates – more reduced=more energy – Long-term storage vs short-term storage – Lipids are essential for structure in ALL organisms: membrane phospholipids • Catabolism of fatty acids –produces acetyl-CoA –produces reducing