Choroid Plexus Tumours: Elucidating the Genomic Complexity of Rare Paediatric Brain Tumours
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Choroid Plexus Papilloma Causing CSF Shunt Ascites: a Rare Presentation
Case Report Annals of Clinical Case Reports Published: 13 Jun, 2017 Choroid Plexus Papilloma Causing CSF Shunt Ascites: A Rare Presentation Deepak Sachan* Department of Pediatrics, Postgraduate Institute of Medical Education and Research, Dr. Ram Manohar Lohia Hospital,New Delhi, India Abstract Choroid Plexus Papillomas (CPPs) are congenital intracranial tumors of neuro-ectodermal origin. Choroid plexus neoplasms constitute about 0.5% of all intracranial neoplasms.Majority are found in lateral ventricles. Most of these neoplasms are benign papillomas, while one-fifth are malignant carcinomas. The present communication describes a rare case of a choroid plexus papilloma leading to CSF ascites following Ventriculoperitoneal (VP) shunt. Case Presentation A 5 year old boy presented to us with complaints of progressively increasing abdominal distension from past 6 months and respiratory distress for 2 days. There was no history of jaundice or bleeding manifestations. Patient was a known case of hydrocephalus for which medium pressure VP shunt (chabra shunt) was placed at the age of 3 years. On examination child was having massive ascitis with positive fluid thrill sign. There was no hepato-spenomegaly and other signs of hepatocellular failure. Neurologically the child was conscious and oriented and there were no signs of shunt dysfunction. Shunt bulb was palpable and soon gets refilled after compressing the bulb. Paracentesis showed clear transudate fluid with no evidence of infection (WBC= 5 cells/mm3, all lymphocytes, sugar = 72 mg/dl and protein = 24 mg/dl). Ascitic fluid culture was sterile and was negative for Acid fast bacilli. In addition, cytology was negative for malignant cell. Liver and renal function test were essentially normal (serum bilirubin = 0.6 mg/dl, SGOT = 36 U/I, SGPT = 11 U/I, serum albumin = 3.8 gm/dl, urea = 27 mg/dl, creatine = 0.8 mg/dl). -

Choroid Plexus Papilloma Arising from the Temporal Horn with a Bilateral Hypersecretory Hydrocephalus: a Case Report and Review of Literature
Elmer ress Case Report World J Oncol. 2016;7(2-3):51-56 Choroid Plexus Papilloma Arising From the Temporal Horn With a Bilateral Hypersecretory Hydrocephalus: A Case Report and Review of Literature Sureswar Mohantya, Suman Saurav Routb, d, Gouri Sankar Sarangia, Kumudini Devic Abstract occur in the third ventricle. These tumors are benign histologi- cally and are neuroectodermal in origin and assigned a WHO Cerebrospinal fluid (CSF) within the cerebral ventricular system is grade I. Complete or gross total resection of these tumors often secreted by a neuroepithelial tissue which is called as the choroid results in a cure and almost recurrence free survival. The spe- plexus. Tumors arising from these tissues are rare. Choroid plexus cial challenges in the management of these tumors are mostly papillomas (CPPs) have been denoted as WHO grade I of the cho- due to its several unique features which include the young age roid plexus tumors. Among the intracranial tumors, neoplasms of the at presentation, high vascularity of these tumors and the poten- choroid plexus constitute around 0.36-0.6%. CPPs are mostly slow tial for hypersecretion of CSF. growing and cause symptoms due to mass effect and obstructive hy- drocephalus, resulting in increased intracranial pressure. We report a Case Report case of CPP arising from the temporal horn in a 7-year-old girl pre- senting with progressive head enlargement since birth due to bilateral massive hydrocephalus without any obstruction, making it purely a A 7-year-old girl presented with progressive head enlargement hypersecretory hydrocephalus. A drainage procedure followed by since birth, features of raised intracranial pressure in the form complete tumor resection was carried out in our case and the patient of headache, vomiting, excessive crying and excessive drowsi- showed marked relief from her symptoms. -

University of California, Irvine
UNIVERSITY OF CALIFORNIA, IRVINE Demystifying the Choroid Plexus THESIS submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in Biomedical Engineering by Esmeralda Romero Lorenzo Thesis Committee: Professor Edwin Monuki, Chair Professor Michelle Digman Professor Wendy Liu 2020 © 2020 Esmeralda Romero Lorenzo TABLE OF CONTENTS LIST OF FIGURES iv LIST OF TABLES v ABSTRACT vii CHAPTER 1: Introduction 1 1.1 The Choroid Plexus and Its functional Role in the Brain 1 1.2 Clinical Significance 3 CHAPTER 2: Choroid Plexus Overview 5 2.1 Anatomy of the Choroid Plexus 5 2.2 Choroid Plexus Epithelial Cells 6 2.3 Choroid Plexus Epithelial Cell Proteins 6 2.3.1 Transthyretin 6 2.3.2 Aquaporin 1 7 2.3.3 ZO-1 8 CHAPTER 3: TTR:tdTomato CPEC Reporter Mouse Culture 9 3.1 TTR:tdTomato Reporter Mouse Characteristics 9 3.2 Methods to Evaluate TTR:tdTomato CPEC Reporter Mouse Line 10 3.2.1. Determining Homozygosity 10 3.2.2 Dissection and Imaging 11 3.3 TTR:tdTomato Reporter Mouse Results 11 CHAPTER 4: Choroid Plexus Epithelial Cell Heterogeneity in vitro 12 4.1 Cell Morphology Heterogeneity in vitro 12 4.2 Methods to Study Heterogeneity in vitro 12 4.2.1 Dissection and Cell Culture 13 4.2.2 Immunohistochemistry 13 4.3 Results 13 4.3.1 Nucleus Size in vitro 14 4.3.2 Cell Morphology 15 4.3.3 Cell Nuclear-Cytoplasmic Ratio 15 4.4 Summary and Future Work 19 CHAPTER 5: CPEC Protein Expression 21 5.1 Heterogeneity in CPEC Protein Expression Heterogeneity 21 5.2 Cell Expression Heterogeneity Methods 21 5.2.1 Dissection and Cell -

Choroid Plexus Tumors: a Review
UC San Diego UC San Diego Previously Published Works Title Perinatal (fetal and neonatal) choroid plexus tumors: a review. Permalink https://escholarship.org/uc/item/0sm7q5q7 Journal Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery, 35(6) ISSN 0256-7040 Authors Crawford, John R Isaacs, Hart Publication Date 2019-06-01 DOI 10.1007/s00381-019-04135-x Peer reviewed eScholarship.org Powered by the California Digital Library University of California Child's Nervous System (2019) 35:937–944 https://doi.org/10.1007/s00381-019-04135-x REVIEW ARTICLE Perinatal (fetal and neonatal) choroid plexus tumors: a review John R. Crawford1,2,3 & Hart Isaacs Jr3,4 Received: 13 September 2018 /Accepted: 20 March 2019 /Published online: 5 April 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Introduction The object of this review is to describe the choroid plexus tumors (CPTs) occurring in the fetus and neonate with regard to clinical presentation, location, pathology, treatment, and outcome. Materials and methods Case histories and clinical outcomes were reviewed from 93 cases of fetal and neonatal tumors obtained from the literature and our own institutional experience from 1980 to 2016. Results Choroid plexus papilloma (CPP) is the most common tumor followed by choroid plexus carcinoma (CPC) and atypical choroid plexus papilloma (ACPP). Hydrocephalus and macrocephaly are the presenting features for all three tumors. The lateral ventricles are the main site of tumor origin followed by the third and fourth ventricles, respectively. CPTs of the fetus are detected most often near the end of the third trimester of pregnancy by fetal ultrasound. -

Superior Parietal Lobule Approach for Choroid Plexus Papillomas Without Preoperative Embolization in Very Young Children
PEDIATRICS CLINICAL ARTICLE J Neurosurg Pediatr 16:101–106, 2015 Superior parietal lobule approach for choroid plexus papillomas without preoperative embolization in very young children Benjamin C. Kennedy, MD,1 Michael B. Cloney, BA,1 Richard C. E. Anderson, MD,1,2 and Neil A. Feldstein, MD1,2 1Department of Neurological Surgery and 2Children’s Hospital of New York, Columbia University, New York, New York OBJECT Choroid plexus papillomas (CPPs) are rare neoplasms, often found in the atrium of the lateral ventricle of infants, and cause overproduction hydrocephalus. The extensive vascularity and medially located blood supply of these tumors, coupled with the young age of the patients, can make prevention of blood loss challenging. Preoperative emboli- zation has been advocated to reduce blood loss and prevent the need for transfusion, but this mandates radiation expo- sure and the additional risks of vessel injury and stroke. For these reasons, the authors present their experience using the superior parietal lobule approach to CPPs of the atrium without adjunct therapy. METHODS A retrospective review was conducted of all children who presented to Columbia University/Morgan Stanley Children’s Hospital of New York with a CPP in the atrium of the lateral ventricle and who underwent surgery using a su- perior parietal lobule approach without preoperative embolization. RESULTS Nine children were included, with a median age of 7 months. There were no perioperative complications or new neurological deficits. All patients had intraoperative blood loss of less than 100 ml, with a mean minimum hematocrit of 26.9% (range 19.6%–36.2%). No patients required a blood transfusion. -

Sv4oearly Region and Large T Antigen in Human Brain Tumors
ICANCER RESEARCH56. 4820-4825. October 5. 1996j SV4O Early Region and Large T Antigen in Human Brain Tumors, Peripheral Blood Cells, and Sperm Fluids from Healthy Individuals' Fernanda Martini, Laura Iacchen, Lorena Lazzarin, Paolo Carinci, Aifredo Corallini, Massimo Gerosa, Paolo luzzolino, Giuseppe Barbanti-Brodano, and Mauro Tognon2 Institute of Histology and General Embryology. (F. M., L 1., L L, P. C.. M. TI, Interdepartment Center for Biotechnology (L I., A. C.. C. B-B., M. TI, and institute of Microbiology (A. C.. G. B-B.]. School of Medicine, Unis'ersitv of Ferrara. Via Fossato di Mortara 64/B, 44100 Ferrara, italy; Department of Neurosurgery. University of Verona (M. G.]. and Pathological Anatomy Sers'ice,Geriatric Hospital of Verona(P. 1.], 37100 Verona.Italy ABSTRACT tected in human brain tumors and in normal brain tissue by Southern blotting and PCR (4—7).JCV sequences were found associated with SV4OT antigen (Tag) coding sequences were detected by PCR ampli normal brain tissue (6) but were not detected in brain tumors in two fication followed by Southern blot hybridization in human brain tumors different investigations (4, 5). Recently, SV4O sequences were de and tumor cell lines, as well as in peripheral blood cells and sperm flUids tected by PCR in ependymomas and choroid plexus papillomas of of healthy donors. SV4Oearly region sequences were found in 83% of choroid plexus papillomas, 73% of ependymomas, 47% of astrocytomas, childhood, as well as in other brain tumors, and in pleural mesothe 33% of glioblastoma multiforme cases,14% of meningiomas,50% of liomas (8—10).SV4O is an infectious agent for monkeys and does not glioblastoma cell lines, and 33% of astrocytoma cell lines and in 23% of normally infect humans. -

Idiopathic Intracranial Hypertension: Any Light on the Mechanism of the Raised Pressure?
J Neurol Neurosurg Psychiatry 2001;71:1–7 1 EDITORIAL Idiopathic intracranial hypertension: any light on the mechanism of the raised pressure? Everyone knows that no one knows the mechanism of the compensatory processes are no longer functioning. Thus increase of intracranial pressure in idiopathic intracranial an increase in cerebral volume with an equivalent hypertension (IIH; also called pseudotumour cerebri; see reduction in CSF volume will obviously not change the table 1 for diagnostic criteria). Does it much matter? After status quo. Over the years investigational techniques of all, for most aVected people IIH is a benign, self limiting every imaginable degree of complexity and invasiveness condition. However, sometimes it is not,1 and current have been used to explore these possibilities in IIH. Many therapies are unsatisfactory. Medical treatment is poor and of the relevant indices such as CSF formation rate, CSF of unproved benefit.23 Surgical interventions (optic nerve outflow resistance, CSF outflow rate, and sagittal sinus sheath fenestration, lumboperitoneal shunting) have ap- pressure can be measured or calculated, but some of the preciable hazards and failure rates.4–10 Moreover, the techniques used require certain assumptions and are mechanism of increase in intracranial pressure in IIH therefore possibly fallible. Particular diYculties exist in might have relevance to raised intracranial pressure and its knowing to what extent the brain is compressible in management in other situations such as meningitis and response to increasing CSF pressure, and to what extent hydrocephalus. the CSF space is expandable. These factors influence CSF outflow resistance calculations in infusion or perfusion Normal intracranial pressure studies. -

CSF Rhinorrhea: a Rare Clinical Presentation of Choroid Plexus Papilloma
Case Report CSF Rhinorrhea: A Rare Clinical Presentation of Choroid Plexus Papilloma Layth Mula-Hussain 1,* , Julia Malone 1, Marlise P. dos Santos 2, John Sinclair 3 and Shawn Malone 1 1 Radiation Oncology Division, The Ottawa Hospital—University of Ottawa, Ottawa, ON K1H 8L6, Canada; [email protected] (J.M.); [email protected] (S.M.) 2 Department of Medical Imaging, The Ottawa Hospital—University of Ottawa, and Ottawa Hospital Research Institute, Ottawa, ON K1H 8L6, Canada; [email protected] 3 Neuro-Surgery Division, The Ottawa Hospital—University of Ottawa, Ottawa, ON K1Y 4E9, Canada; [email protected] * Correspondence: [email protected] Received: 6 November 2020; Accepted: 27 January 2021; Published: 31 January 2021 Abstract: Choroid plexus papilloma (CPP) is a rare brain tumour occurring mostly in infants and children. Most CPPs are intraventricular and present with symptoms and signs of increased intracranial pressure (ICP). This case report describes a middle-aged female who presented with spontaneous cerebrospinal fluid (CSF) rhinorrhea from a tumour located in the cerebellopontine angle (CPA). She underwent craniotomy with subtotal tumour resection and remained progression and rhinorrhea-free for several years. Upon clinical progression, the patient was treated with Cyberknife stereotactic radiosurgery. The patient clinically improved and demonstrated a favourable radiologic response to radiosurgery. Keywords: choroid plexus tumour; cerebellopontine angle; CSF rhinorrhea; stereotactic radiosurgery 1. Introduction Choroid plexus tumour (CPT) is a rare epithelial intracranial extra-axial brain tumour that usually grows inside ventricles and is connected to the choroid plexus or near the natural openings of the ventricles. The annual incidence is 0.3 cases per million [1], and they account for about 0.5% of all intracranial neoplasms. -

Parapharyngeal Space Tumors
IJHNS 10.5005/jp-journals-10001-1059 REVIEW ARTICLE Parapharyngeal Space Tumors Parapharyngeal Space Tumors Pratima S Khandawala Senior Registrar, Department of ENT, Holy Family Hospital, Bandra, Mumbai, Maharashtra, India Correspondence: Pratima S Khandawala, Senior Registrar, Department of ENT, Holy Family Hospital, Bandra, Mumbai Maharashtra, India, e-mail: [email protected] ABSTRACT Parapharyngeal space is a potential space in the neck extending from skull base to the greater cornu of hyoid bone. It is divided in prestyloid and poststyloid compartment by the fascia joining styloid process to tensor veli palatini. Tumors of parapharyngeal space are uncommon, comprising of less than 1% of all head and neck neoplasms. CT Scanning and MRI investigations is complimentary and both studies should be performed for evaluation of lesions in this area. Complete surgical excision is the mainstay of treatment. Keywords: Parapharyngeal space, Prestyloid, Poststyloid, CT scan, MRI. ANATOMY It is continuous with the retropharyngeal space and also communicates with other cervical and cranial fascial spaces The parapharyngeal space (or lateral pharyngeal space) is a as well as the mediastinum. potential space in the neck shaped like an inverted pyramid. Divisions and Contents (Fig. 2) Boundaries (Fig. 1) The parapharyngeal space is divided into prestyloid and • Inferior greater cornu of the hyoid bone forming the apex poststyloid compartments by the fascia joining the styloid • Superior—base of skull (sphenoid and temporal bones), process to the tensor veli palatini. this area includes the jugular and hypoglossal foramen These lymphatics receive afferent drainage from the oral and the foramen lacerum cavity, oropharynx, paranasal sinuses and thyroid. -

Perinatal (Fetal and Neonatal) Astrocytoma: a Review
Childs Nerv Syst DOI 10.1007/s00381-016-3215-y REVIEW PAPER Perinatal (fetal and neonatal) astrocytoma: a review Hart Isaacs Jr.1,2 Received: 16 July 2016 /Accepted: 3 August 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Keywords Fetal astrocytoma . Neonatal astrocytoma . Introduction The purpose of this review is to document the Perinatal astrocytoma . Intracranial hemorrhage . Congenital various types of astrocytoma that occur in the fetus and neo- brain tumor nate, their locations, initial findings, pathology, and outcome. Data are presented that show which patients are likely to sur- vive or benefit from treatment compared with those who are Introduction unlikely to respond. Materials and methods One hundred one fetal and neonatal Glial cells are the supportive elements of the central nervous tumors were collected from the literature for study. system (CNS) [22]. They include astrocytes, oligodendro- Results Macrocephaly and an intracranial mass were the most cytes, and ependymal cells, and the corresponding tumors common initial findings. Overall, hydrocephalus and intracra- originating from these cells astrocytoma, oligodendroglioma, nial hemorrhage were next. Glioblastoma (GBM) was the and ependymoma all of which are loosely called Bglioma^ most common neoplasm followed in order by subependymal [16, 22]. The term Bglioma^ is used interchangeably with giant cell astrocytoma (SEGA), low-grade astrocytoma, ana- astrocytoma to describe the more common subgroup of tu- plastic astrocytoma, and desmoplastic infantile astrocytoma mors [22]. (DIA). Tumors were detected most often toward the end of Glioma (astrocytoma) is the leading CNS tumor in chil- the third trimester of pregnancy. -

Choroid Plexus Papilloma: Case Report Papiloma Do Plexo Coroide: Relato De Caso
CASE REPORT J Bras Patol Med Lab. 2019; 55(6): 675-682. Choroid plexus papilloma: case report Papiloma do plexo coroide: relato de caso Wilker D. Martins; Wander N. Naves 10.5935/1676-2444.20190060 Universidade Federal de Goiás (UFG), Goiânia, Goiás, Brazil. ABSTRACT Choroid plexus papillomas (CPP) are a rare oncological condition. They affect mostly the pediatric population, and the diagnosis is associated with clinical findings, imaging and anatomopathological methods. We report the case of a 49-year-old woman who underwent neurosurgical evaluation for chronic headache and emotional stress. CPP is a rare central nervous system tumor in the adult population. We present, therefore, a case in an adult female, whose diagnosis was confirmed by histopathology methods. Surgical treatment is the gold standard, showing full resolution in almost all cases. Key words: choroid plexus papillomas; hydrocephalus; brain tumors. RESUMO Os papilomas do plexo coroide (PPC) são uma condição oncológica bastante rara. Acometem majoritariamente a população pediátrica; seu diagnóstico envolve uma análise clínica associada aos métodos de imagem e anatomopatológico. Relatamos o caso de uma mulher de 49 anos atendida no Hospital das Clínicas da Universidade Federal de Goiás (UFG) para avaliação neurocirúrgica com clínica de cefaleia crônica e estresse emocional. O PPC é um tumor do sistema nervoso central extremamente raro na população adulta. Apresentamos, portanto, um caso raro em uma mulher adulta, cujo diagnóstico foi confirmado por métodos histopatológicos. O tratamento cirúrgico é o padrão-ouro, pois mostra resolução completa em quase todos os casos. Unitermos: papiloma do plexo coroide; hidrocefalia; tumores cerebrais. RESUMEN Los papilomas de los plexos coroideos (PPC) son una condición oncológica bastante rara. -
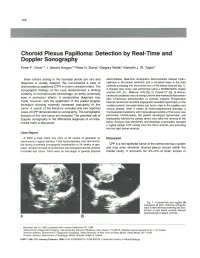
Choroid Plexus Papilloma: Detection by Real-Time and Doppler Sonography
168 Choroid Plexus Papilloma: Detection by Real-Time and Doppler Sonography Peter P. Chow, ·2 J . Gerard Horgan,·3 Peter N. Burns' Gregory Weltin' Kenneth J. W. Taylor' Brain tumors arising in the neonatal period are rare and abnormalities. Real-time sonograms demonstrated marked hydro diagnosis is usually delayed. We encountered a case of cephalus in the lateral ventricles and a lobulated mass in the third choroid plexus papilloma (CPP) in a term newborn infant. The ventricle extending into the frontal horn of the lateral ventricle (fig . 1). sonographic findings of this case demonstrated a striking A Doppler flow study was performed using a Mk600/5MHz duplex scanner (ATL Inc., Bellevue, WA) (fig. 2). Cranial CT (fig . 3) demon similarity to intraventricular hemorrhage, an entity commonly strated an isodense mass showing uniform and marked enhancement seen in premature infants. A preoperative diagnosis was after intravenous administration of contrast material. Preoperative made, however, with the application of the pulsed Doppler internal carotid and vertebral angiograms revealed hypertrophy of the technique showing markedly increased vascularity of this medial posterior choroidal artery and tumor stain in the capillary and tumor. A search of the literature revealed only two reported venous phases. After 3 weeks of ventriculoperitoneal drainage, a cases of CPP demonstrated by sonography. The sonographic frontoparietal craniotomy with transcallosal excision of the tumor was features of this rare tumor are reviewed . The potential role of performed. Unfortunately, the patient developed hypotension and Doppler sonography in the differential diagnosis of an intra bradycardia followed by cardiac arrest soon after the removal of the cranial ma s is discussed.