The Spinal Locomotor Circuit of the Larval Zebrafish: Anatomical Organization and Functional Components
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Single-Neuron: Current Trends and Future Prospects
cells Review A Single-Neuron: Current Trends and Future Prospects Pallavi Gupta 1, Nandhini Balasubramaniam 1, Hwan-You Chang 2, Fan-Gang Tseng 3 and Tuhin Subhra Santra 1,* 1 Department of Engineering Design, Indian Institute of Technology Madras, Tamil Nadu 600036, India; [email protected] (P.G.); [email protected] (N.B.) 2 Department of Medical Science, National Tsing Hua University, Hsinchu 30013, Taiwan; [email protected] 3 Department of Engineering and System Science, National Tsing Hua University, Hsinchu 30013, Taiwan; [email protected] * Correspondence: [email protected] or [email protected]; Tel.: +91-044-2257-4747 Received: 29 April 2020; Accepted: 19 June 2020; Published: 23 June 2020 Abstract: The brain is an intricate network with complex organizational principles facilitating a concerted communication between single-neurons, distinct neuron populations, and remote brain areas. The communication, technically referred to as connectivity, between single-neurons, is the center of many investigations aimed at elucidating pathophysiology, anatomical differences, and structural and functional features. In comparison with bulk analysis, single-neuron analysis can provide precise information about neurons or even sub-neuron level electrophysiology, anatomical differences, pathophysiology, structural and functional features, in addition to their communications with other neurons, and can promote essential information to understand the brain and its activity. This review highlights various single-neuron models and their behaviors, followed by different analysis methods. Again, to elucidate cellular dynamics in terms of electrophysiology at the single-neuron level, we emphasize in detail the role of single-neuron mapping and electrophysiological recording. We also elaborate on the recent development of single-neuron isolation, manipulation, and therapeutic progress using advanced micro/nanofluidic devices, as well as microinjection, electroporation, microelectrode array, optical transfection, optogenetic techniques. -
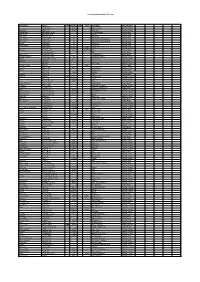
C:\Boatlists\Boatlistdraft-2021.Xlsx Boat Name Owner Prefix Sail No
C:\BoatLists\boatlistdraft-2021.xlsx Boat Name Owner Prefix Sail No. Suffix Hull Boat Type Classification Abraham C 2821 RS Feva XL Sailing Dinghy Dunikolu Adams R 10127 Wayfarer Sailing Dinghy Masie Mary Adlington CPLM 18ft motorboat Motor Boat Isla Rose Adlington JPN Tosher Sailing Boat Demelza Andrew JA 28 Heard 28 Sailing Boat Helen Mary Andrew KC 11 Falmouth Working Boat Sailing Boat Mary Ann Andrew KC 25 Falmouth Working Boat Sailing Boat Verity Andrew N 20 Sunbeam Sailing Boat West Wind Andrew N 21 Tosher 20 Sailing Boat Andrews K 208210 white Laser 4.7 Sailing Dinghy Hermes Armitage AC 70 dark blue Ajax Sailing Boat Armytage CD RIB Motor Boat Alice Rose Ashworth TGH Cockwell's 38 Motor Boat Maggie O'Nare Ashworth TGH 10 Cornish Crabber Sailing Cruiser OMG Ashworth* C & G 221 Laser Pico Sailing Dinghy Alcazar Bailey C Motor Boat Bailey C RS Fevqa Sailing Dinghy Dither of Dart Bailey T white Motor Sailer Coconi Barker CB 6000 Contessa 32 Sailing Cruiser Diana Barker G Rustler 24 Sailing Boat Barker G 1140 RS200 Sailing Dinghy Gemini Barnes E RIB Motor Boat Pelorus Barnes E GBR 3731L Arcona 380 Sailing Cruiser Barnes E 177817 Laser Sailing Dinghy Barnes F & W 1906 29er Sailing Dinghy Lady of Linhay Barnes MJ Catamaran Motor Boat Triumph Barnes MJ Westerly Centaur Sailing Cruiser Longhaul Barstow OG Orkney Longliner 16 Motor Boat Barö Barstow OG 2630 Marieholm IF-Boat Sailing Cruiser Rinse & Spin Bateman MCW 5919 Laser Pico Sailing Dinghy Why Hurry Batty-Smith JR 9312 Mirror Sailing Dinghy Natasha Baylis M Sadler 26 Sailing Cruiser -

2021 Freshwater Fishing Regulations Summary (Except Trout)
License Information Summary of Fishing Regulations 2021 REGULATIONS 2021 REGULATIONS Regulations in red are new this year. permits and stamps. These privileges are not avail- • Fish may be taken only in the manner known as able using Fish and Wildlife’s website. However, the angling with handline or with rod and line, or NJ Dept. of Military and Veterans Affairs can issue as otherwise allowed by law. Licenses fishing licenses through their DMAVA website at • When fishing from the shoreline, no more than • A valid New Jersey fishing license is required for www.nj.gov/military/iasd/fishing.html. For all other three fishing rods, handlines or combination residents at least 16 years and less than 70 years free sporting licenses, call (609) 530-6866, email thereof may be used (except on the Delaware of age (plus all non-residents 16 years and older) [email protected], or write to: MSG (Ret.) River. There is no rod limit when fishing from to fish the fresh waters of New Jersey, including Robert Greco, NJ DMAVA, 101 Eggert Crossing a boat except for the Delaware River.) For the privately owned waters. See page 17 for informa- Rd., Lawrenceville, NJ 08648. Delaware River the three rod limit applies both tion on the money-saving Buddy Fishing License� to boat and shoreline anglers (see page 28). • For fishing-related license and permit fees, see Veterans Licenses, Stamps and Permits • Only one daily creel of any fish species may be in page 1. See below for Purchasing a License. Free hunting and fishing licenses, stamps and possession. -

RSG Book Template 2011 V4 051211
IUCN IUCN, International Union for Conservation of Nature, helps the world find pragmatic solutions to our most pressing environment and development challenges. IUCN works on biodiversity, climate change, energy, human livelihoods and greening the world economy by supporting scientific research, managing field projects all over the world, and bringing governments, NGOs, the UN and companies together to develop policy, laws and best practice. IUCN is the world’s oldest and largest global environmental organization, with more than 1,200 government and NGO members and almost 11,000 volunteer experts in some 160 countries. IUCN’s work is supported by over 1,000 staff in 60 offices and hundreds of partners in public, NGO and private sectors around the world. IUCN Species Survival Commission (SSC) The SSC is a science-based network of close to 8,000 volunteer experts from almost every country of the world, all working together towards achieving the vision of, “A world that values and conserves present levels of biodiversity.” Environment Agency - ABU DHABI (EAD) The EAD was established in 1996 to preserve Abu Dhabi’s natural heritage, protect our future, and raise awareness about environmental issues. EAD is Abu Dhabi’s environmental regulator and advises the government on environmental policy. It works to create sustainable communities, and protect and conserve wildlife and natural resources. EAD also works to ensure integrated and sustainable water resources management, and to ensure clean air and minimize climate change and its impacts. Denver Zoological Foundation (DZF) The DZF is a non-profit organization whose mission is to “secure a better world for animals through human understanding.” DZF oversees Denver Zoo and conducts conservation education and biological conservation programs at the zoo, in the greater Denver area, and worldwide. -

January 2019 No
C A R I B B E A N On-line C MPASS JANUARY 2019 NO. 280 The Caribbean’s Monthly Look at Sea & Shore Women’s Match Racing in St. Thomas See Regatta News on page 11 DEAN BARNES JANUARY 2019 CARIBBEAN COMPASS PAGE 2 BILL THOMAS The Caribbean’s Monthly Look at Sea & Shore www.caribbeancompass.com JANUARY 2019 • NUMBER 280 DEAN BARNESWeather Sources GABY EMAN Online and on SSB .......22 & 27 DEPARTMENTS Annual Calendar Info & Updates ......................4 Meridian Passage .................37 of Events Business Briefs .......................8 The Caribbean Sky ...............38 Caribbean events 2019 ......... 23 Regatta News........................ 11 Cooking with Cruisers ..........37 MONICA PISANI Y2A ......................................... 16 Readers’ Forum .....................38 Island Poets ...........................33 Caribbean Market Place .....42 Book Review ......................... 34 Calendar of Events ...............45 Big Up! Look Out For… ......................35 Classified Ads ....................... 46 USVI Show largest in years ...10 Seaboard Birds ..................... 36 Advertisers Index ..................46 Caribbean Compass is published monthly by Compass Publishing Ltd., The Valley, P.O. Box 727, JANUARY 2019 CARIBBEAN COMPASS PAGE 3 Anguilla, British West Indies. Tel: (784) 457-3409, Fax: (784) 457-3410, [email protected], www.caribbeancompass.com Publisher..................................Tom Hopman Art, Design & Production.........Wilfred Dederer [email protected] [email protected] Editor...........................................Sally -

Autumn 07 Cover
12 June 2012 (First Session, Lots 1–455) Miscellaneous and Mixed Lots 9 First Session, Lots 1– 455 Tuesday 12th June at 11 am Miscellaneous and Mixed Lots 1 A Q.V. to K.G.V collection in an Imperial album, incl. Australian States, Barbados, Canada 1897 Jubilee to 50c. mint, Cyprus, Gibraltar, Great Britain 1840 1d. (2) 2d. (2) used, 1862-64 3d. unused, 1887-92 to 1s. (both) mint, 1913 £1 used, Hong Kong with 1912-21 $10 used, British P.O’s in China 1917-21 $5 mint, India and States, Ireland 1935 re-engraved set mint, Kenya, Uganda and Tanganyika 1922-27 to £1 mint, Labuan, Malayan States with Straits Settlements, Malta 1886 5s., mint, 1899-1901 set mint, 1926-27 set mint, Mauritius, Newfoundland, New Zealand, North Borneo, Rhodesia 1910-13 2s.6d. used, Saint Helena, Sarawak, Sierra Leone 1896-97 £1 mint, South Africa with Cape of Good Hope Mafeking 1900 3d. unused, etc., mixed condition. (many 100s) £9,000-£10,000 2 A mainly Q.V. to Q.E.II collection in six albums, incl. Brunei with 1922 Malaya-Borneo set mint, Great Britain with 1840 1d. black (6), 2d. blue two pairs used, 1870 ½d. plate 9 used, 1887-92 Jubilee £1 green used, 1913 Waterlow £1 green used, India with 1911-22 to 25r. used, 1929 Air 8a. with variety missing tree top mint, 1937-40 to 25r. used, Officials, Convention States with Chamba, Indian Feudatory States with Barwani, Bhopal, Bundi, Cochin, Hyderabad, Jaipur, Malaya and States with Straits Settlements 1867 4c. -

Newletters 2006
The NEWSLETTER International Hydrofoil Society P. O. Box 51, Cabin John MD 20818 USA Editor: John R. Meyer Spring 2006 SEABUS-HYDAER: WING SUSTAINING MEMBERS ASSISTED HYDROFOIL oday there is a general need to transport passengers, cars and cargo Tby sea using methods that are safe, fast and cost-effective. European research is developing new types of ship to fulfill this demand. AEu- ropean project, SEABUS-HYDAER, is developing a completely new concept in marine transport: a hybrid between a plane and a ship. Al- though Seabus never leaves the water, most of its lift power is provided by its wings. This gives it the capacity to travel very fast. Ferries cur- rently in use have top speed of about 40 knots; Seabus beats this with a ______________________________ projected speed of about 120 knots (220 km/h) while using 20% less fuel. 2006 DUES ARE DUE Shown here are several renderings of the HYDAER concept. IHS Membership is still only US$20 per calendar year (US$10 for stu- dents). For payment of regular member- ship dues by credit card using PAYPAL., please go to the IHS membership page at <http://www.foils.org/member.htm> and follow the instructions. INSIDE THIS ISSUE - President’s Column -----------p. 2 - Welcome New Members -----p. 2 - Capt. Peterson Honored----- p. 4 - Foilmasters -------------------- p. 4 Rendering of the Seabus-Hydaer Concept - Foil Kayak ----------------------p. 6 The aim of the European project Seabus-Hydaer is the development of a new generation of Fast Ferries (>100 knots). Supramar is providing the - ASNE Day 2006 --------------- p. 9 help through the investigation of specific hydrofoils for stabilisation and - Sailor’s Page ------------------ p. -

Sing! 1975 – 2014 Song Index
Sing! 1975 – 2014 song index Song Title Composer/s Publication Year/s First line of song 24 Robbers Peter Butler 1993 Not last night but the night before ... 59th St. Bridge Song [Feelin' Groovy], The Paul Simon 1977, 1985 Slow down, you move too fast, you got to make the morning last … A Beautiful Morning Felix Cavaliere & Eddie Brigati 2010 It's a beautiful morning… A Canine Christmas Concerto Traditional/May Kay Beall 2009 On the first day of Christmas my true love gave to me… A Long Straight Line G Porter & T Curtan 2006 Jack put down his lister shears to join the welders and engineers A New Day is Dawning James Masden 2012 The first rays of sun touch the ocean, the golden rays of sun touch the sea. A Wallaby in My Garden Matthew Hindson 2007 There's a wallaby in my garden… A Whole New World (Aladdin's Theme) Words by Tim Rice & music by Alan Menken 2006 I can show you the world. A Wombat on a Surfboard Louise Perdana 2014 I was sitting on the beach one day when I saw a funny figure heading my way. A.E.I.O.U. Brian Fitzgerald, additional words by Lorraine Milne 1990 I can't make my mind up- I don't know what to do. Aba Daba Honeymoon Arthur Fields & Walter Donaldson 2000 "Aba daba ... -" said the chimpie to the monk. ABC Freddie Perren, Alphonso Mizell, Berry Gordy & Deke Richards 2003 You went to school to learn girl, things you never, never knew before. Abiyoyo Traditional Bantu 1994 Abiyoyo .. -

2007 Texas Reading Club Manual Sail Away with Books!
Sail Away With Books! 1 2007 Texas Reading Club Manual 2007 Texas Reading Club Manual Sail Away With Books! Written By: Barrie Alguire, Miranda Bauer, Linda Befeld, Sara Bracht, Debbie Brightwell Brown, Lucina Carpenter, Paola Ferate-Soto, WyLaina Hildreth, Shelly Lane, and Christine McNew Clip Art By: Diane Stanley and Shawn Clements Theme Songs By: Lucas Miller and Sally Meyers Craft Patterns and Illustrations By: Shawn Clements Edited By: Jeanette Larson and Christine McNew Published By: The Library Development Division of the Texas State Library and Archives Commission, Austin, Texas 2007 2 Sail Away With Books! Table Of Contents Preface 4 Something About the Artist 5 Acceptable Use of Artwork 6 Something About the Authors and Songwriters 8 Introduction 11 Theme Songs 34 Reader's Theater Scripts 38 Puppet Plays 47 Stories 57 Celebrations Programs Chapter 60 Family Program 76 Bilingual Programs Chapter 85 Toddler Programs Chapter 146 Preschool Programs Chapter 206 Elementary Programs Chapter 278 Young Adult Programs Chapter 320 Bibliography 364 3 2007 Texas Reading Club Manual Preface This manual is created for the 2007 Texas Reading Club, a program of the Library Development Division of the Texas State Library and Archives Commission. The theme is “Sail Away with Books!” in English and “¡Navega lejos con libros!” in Spanish. The programs invite children to explore oceans, lakes, boats, voyages, water sports, pirates, and many related topics. The 2007 Texas Reading Club manual is available on CD-ROM and online at the Texas State Library web site, www.tsl.state.tx.us/ld/projects/trc/2007/index.html. Children’s book illustrator, Diane Stanley, created the artwork for the 2007 Texas Reading Club. -

To the Point
To the Point the small point summer school newsletter spring 2010 contents President’s Letter A Note from the Directors Drama 2010 Season Preview Tennis Sailing Development and Fundraising A Garden Grows at SPSS Community Calendar Community Directory from the director Transforming Tadpole President’s Letter Newsletter News ello small pointers and SPSS campers! As anyone knows who’s been out to Newsletter Notes the Point (or logged on to the smallpoint.org webcam, or looked at recent pictures H of the beach or Club Road) it’s been a wild, wild winter. Snow and ice, wind and rain, waves and historically high tides have all had their way with our little corner of the coast, and the Summer School grounds were not spared. Merritt and Buell Heminway, newsletter notes Grey Terry and John Herrigel recently pitched in to right the Tadpole after she blew off This issue of To the Point is being her stands! distributed exclusively in digital Our intrepid new directors, Ben Lewis and John Herrigel, have stepped fearlessly form. This is more efficient, less into Malcolm’s big shoes. They’ve been hard at work recruiting counselors, figuring out expensive, and far more environ- budgets, developing exciting new programs and tweaking and improving old favorites. mentally friendly than printing and We think campers young and old and their families will be delighted with this summer’s mailing. However, we realize that full roster of activities. Highlights include an expansion of last year’s very popular some people will not be able to advanced sailing and regatta program, new community movie nights at Brad’s Barn, and receive the newsletter digitally. -

LYNCHES Scenic River Water Trail Guide Images L the Photographs Used in This Publication Were Taken by South Carolina Department of Natural Resources Staff
LYNCHES Scenic River Water Trail Guide Images l The photographs used in this publication were taken by South Carolina Department of Natural Resources staff. l All historic map images were provided by the Alabama University Historical Map and GIS Department. l The photographs of the historic bridges along the Lynches River were provided by South Carolina Archives and History, South Carolina Cotton Museum and Ralph Des Champs. l The photographs from South Carolina Archives and History may be found in file number S233W2 of the collection provided by the South Carolina Department of Transportation. l Waste product images were taken from the Internet and used with permission of the product companies. Contents The Lynches River ..........................................................................1 The South Carolina Scenic Rivers Program ......................2 River Conservation ........................................................................3 Facts about the Lynches River .................................................4 Landings and River Miles .............................................................5 Streamflow Information ..............................................................6 Approximate One-Way Shuttle Information ....................................7 Natural History ............................................................................. 13 Geology and Fluvial Geomorphology ................................... 13 Flora ............................................................................................. -

Boating & Sailing
02 4354-2 CH01 2/21/02 12:46 PM Page 3 Chapter1 Welcome to the Wet and Wonderful World of Boating! In This Chapter N What kind of boat is right for you? N Learning the vocabulary of boating N I’m not your type—classes of boats N If you have to ask how much it costs … you’re smart! N Buying your first boat: New or used? You’ve looked at the boating magazines and mooned around the boat shows long enough. Now it’s time for you to be the guy or gal next to that smiling, Florida-tanned model with the mirrored sunglasses and the wind-rippled hair, cruising across that emerald sea toward adventure, romance, and a monthly payment book. Or maybe your vision is a bit more domestic: the spouse, the kids, the Boston terrier, a jug of Gatorade, and a picnic basket full of pastrami on rye, off for a 02 4354-2 CH01 2/21/02 12:46 PM Page 4 4 Part 1: Getting Started in Boating visit to a sandy beach. Sailing, water-skiing, snorkeling, a visit to the next port down the river, or maybe a cruise to that island over the horizon—you are the skipper; you decide. You’re in command in a world where there are no yellow lines and no traffic jams, where fish and birds and wonderful marine mammals such as porpoise and manatees are fre- quently part of the scenery, and where you can find true solitude when you want it. No wonder people love boats and boating.