Lipid Management in Patients with Endocrine Disorders: an Endocrine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

The Differential Effects of Statins on the Metastatic Behaviour of Prostate Cancer
British Journal of Cancer (2012) 106, 1689–1696 & 2012 Cancer Research UK All rights reserved 0007 – 0920/12 www.bjcancer.com The differential effects of statins on the metastatic behaviour of prostate cancer *,1 1 1 2,3 2,3 2,4 1,2,4 M Brown , C Hart , T Tawadros , V Ramani , V Sangar , M Lau and N Clarke 1 Genito Urinary Cancer Research Group, University of Manchester, Paterson Institute for Cancer Research, Manchester Academic Health Science Centre, 2 The Christie NHS Foundation Trust, Wilmslow Road, Withington, Manchester M20 4BX, UK; Department of Urology, The Christie NHS Foundation 3 Trust, Wilmslow Road, Manchester M20 4BX, UK; Department of Urology, University Hospital of South Manchester NHS Trust, Manchester M23 9LT, 4 UK; Department of Urology, Salford Royal NHS Foundation Trust, Stott Lane, Manchester M6 8HD, UK BACKGROUND: Although statins do not affect the incidence of prostate cancer (CaP), usage reduces the risk of clinical progression and mortality. Although statins are known to downregulate the mevalonate pathway, the mechanism by which statins reduce CaP progression is unknown. METHODS: Bone marrow stroma (BMS) was isolated with ethical approval from consenting patients undergoing surgery for non- malignant disease. PC-3 binding, invasion and colony formation within BMS was assessed by standardised in vitro co-culture assays in the presence of different statins. RESULTS: Statins act directly on PC-3 cells with atorvastatin, mevastatin, simvastatin (1 mM) and rosuvastatin (5 mM), but not pravastatin, significantly reducing invasion towards BMS by an average of 66.68% (range 53.93–77.04%; Po0.05) and significantly reducing both 2 2 number (76.2±8.29 vs 122.9±2.48; P ¼ 0.0055) and size (0.2±0.0058 mm vs 0.27±0.012 mm ; P ¼ 0.0019) of colonies formed within BMS. -
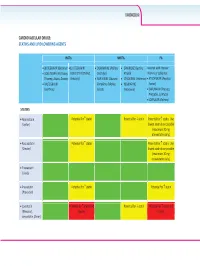
Statins and Lipid Lowering Agents
CARDIOVASCULAR Z/Ks^h>ZZh'^͗ ^dd/E^E>/W/>KtZ/E''Ed^ /E^d/Ɛ EEZd/Ɛ W/Ɛ x/d'Zs/Z ;ŝŬƚĂƌǀLJͿ x>s/d'Zs/Zͬ x KZs/Z/E ;WŝĨĞůƚƌŽ͕ x &s/ZE ;^ƵƐƚŝǀĂ͕ ŽŽƐƚĞĚǁŝƚŚƌŝƚŽŶĂǀŝƌ xK>hd'Zs/Z ;dŝǀŝĐĂLJ͕ K//^dd ;^ƚƌŝďŝůĚ͕ ĞůƐƚƌŝŐŽͿ ƚƌŝƉůĂͿ ;EŽƌǀŝƌͿŽƌĐŽďŝĐŝƐƚĂƚ dƌŝƵŵĞƋ͕:ƵůƵĐĂ͕ŽǀĂƚŽͿ 'ĞŶǀŽLJĂͿ x Z/>W/s/Z/E ;ĚƵƌĂŶƚ͕ x dZs/Z/E ;/ŶƚĞůĞŶĐĞͿ xdEs/Z ;ZĞLJĂƚĂnj͕ xZ>d'Zs/Z ŽŵƉůĞƌĂ͕KĚĞĨƐĞLJ͕ x Es/ZW/E ǀŽƚĂnjͿ ;/ƐĞŶƚƌĞƐƐͿ :ƵůƵĐĂͿ ;sŝƌĂŵƵŶĞͿ xZhEs/Z ;WƌĞnjŝƐƚĂ͕ WƌĞnjĐŽďŝdž͕^LJŵƚƵnjĂͿ x>KW/Es/Z ;<ĂůĞƚƌĂͿ ^dd/E^ xƚŽƌǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;>ŝƉŝƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϮϬŵŐ ĂƚŽƌǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xZŽƐƵǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;ƌĞƐƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϭϬŵŐ ƌŽƐƵǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xWŝƚĂǀĂƐƚĂƚŝŶ ;>ŝǀĂůŽͿ xWƌĂǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ ;WƌĂǀĂĐŚŽůͿ x>ŽǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ ;DĞǀĂĐŽƌͿ͕ ƚŽdžŝĐŝƚLJ ƚŽdžŝĐŝƚLJ ƐŝŵǀĂƐƚĂƚŝŶ ;ŽĐŽƌͿ CARDIOVASCULAR INSTIs NNRTIs PIs xBICTEGRAVIR (Biktarvy) xELVITEGRAVIR/ x DORAVIRINE (Pifeltro, x EFAVIRENZ (Sustiva, Boosted with ritonavir xDOLUTEGRAVIR (Tivicay, COBICISTAT (Stribild, Delstrigo) Atripla) (Norvir) or cobicistat Triumeq, Juluca) Genvoya) x RILPIVIRINE (Edurant, x ETRAVIRINE (Intelence) xATAZANAVIR (Reyataz, xRALTEGRAVIR Complera, Odefsey, x NEVIRAPINE Evotaz) (Isentress) Juluca) (Viramune) xDARUNAVIR (Prezista, Prezcobix, Symtuza) xLOPINAVIR (Kaletra) FIBRATES xFenofibrate, bezafibrate, gemfibrozil CHOLESTEROL ABSORPTION INHIBITOR xEzetimibe -

Critical Impact of Drug-Drug Interactions Via Intestinal CYP3A in the Risk Assessment of Weak Perpetrators Using Physiologically Based Pharmacokinetic Models
DMD Fast Forward. Published on January 29, 2020 as DOI: 10.1124/dmd.119.089599 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 89599 Title page Critical impact of drug-drug interactions via intestinal CYP3A in the risk assessment of weak perpetrators using physiologically based pharmacokinetic models Makiko Yamada, Shin-ichi Inoue, Daisuke Sugiyama, Yumi Nishiya, Tomoko Ishizuka, Akiko Watanabe, Downloaded from Kengo Watanabe, Shinji Yamashita, Nobuaki Watanabe Drug Metabolism and Pharmacokinetics Research Laboratories, Daiichi Sankyo Co., Ltd., Tokyo, Japan dmd.aspetjournals.org (M.Y., S.I., D.S., Y.N., T.I., A.W., K.W., N.W.) and Faculty of Pharmaceutical Sciences, Setsunan University (S.Y.) at ASPET Journals on October 1, 2021 1 DMD Fast Forward. Published on January 29, 2020 as DOI: 10.1124/dmd.119.089599 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 89599 Running title page Running title: PBPK modeling of weak perpetrators via intestinal CYP3A *Corresponding author: Makiko Yamada, Drug Metabolism and Pharmacokinetics Research Laboratories, Daiichi Sankyo Co., Ltd., 1-2-58, Hiromachi, Shinagawa-ku, Tokyo 140-8710, Japan. Phone: +81-3-3492- Downloaded from 3131; Fax: +81-3-5436-8567. E-mail: [email protected] Number of text pages: 28 Number of tables: 3 dmd.aspetjournals.org Number of figures: 5 Number of references: 23 Number of words in the Abstract: 245 at ASPET Journals on October 1, 2021 Number of words in the Introduction: 706 Number of words in the Discussion: 1483 Abbreviations: ACAT, Advanced Compartmental Absorption and Transit; AUC, area under the curve; AUCR, AUC ratio; CYP3A, cytochrome P450 3A; DDI, drug-drug interaction; FDA, Food and Drug Administration; Fg, intestinal availability; FPE, first pass effect; GMFE, geometric mean fold error ; LC- MS/MS, liquid chromatography-tandem mass spectrometry; PBPK, physiologically based pharmacokinetic; TDI, time-dependent inhibition. -

Effect of the Nutritional Supplement Alanerv on the Serum PON1 Activity in Post-Acute Stroke Patients
Pharmacological Reports Copyright © 2013 2013, 65, 743750 by Institute of Pharmacology ISSN 1734-1140 Polish Academy of Sciences Shortcommunication EffectofthenutritionalsupplementALAnerv® ontheserumPON1activityinpost-acutestroke patients BogdanN.Manolescu1,MihaiBerteanu2,DeliaCintezã2 1 DepartmentofOrganicChemistry”C.Nenitescu”,FacultyofAppliedChemistryandScienceofMaterials, PolytechnicUniversityofBucharest,011061,Bucharest,Romania 2 DepartmentofRehabilitationandPhysicalMedicine,FacultyofMedicine,UniversityofMedicineandPharmacy ”CarolDavila”,020022,Bucharest,Romania Correspondence: BogdanN.Manolescu,e-mail:[email protected] Abstract: Background: Paraoxonase-1 (PON1) is one of the HDL-associated proteins which contributes to the antioxidant properties of these lipoproteins. The aim of this pilot study was to evaluate the effect of the nutritional supplement ALAnerv® on serum PON1 activity inpost-acutestrokepatientsundergoingrehabilitation. Methods: We enrolled 28 post-acute stroke patients and randomly divided them into (–) ALA or (+) ALA study groups. All the pa- tients underwent the same rehabilitation program and received comparable standard medications. Moreover, (+) ALA patients re- ceived ALAnerv® for two weeks (2 pills/day). The serum PON1 activity was assessed on blood samples taken at the admission and at the discharge moments, respectively. We used paraoxon (paraoxonase activity, PONA), phenyl acetate (arylesterase activity, ARYLA) and dihydrocoumarin (lactonase activity, LACTA) as substrates, the latter activity being regarded -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Clinofibrate Improved Canine Lipid Metabolism in Some but Not All Breeds
NOTE Internal Medicine Clinofibrate improved canine lipid metabolism in some but not all breeds Yohtaro SATO1), Nobuaki ARAI2), Hidemi YASUDA3) and Yasushi MIZOGUCHI4)* 1)Graduate School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan 2)Spectrum Lab Japan, 1-5-22-201 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 3)Yasuda Veterinary Clinic, 1-5-22 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 4)School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan ABSTRACT. The objectives of this study were to assess if Clinofibrate (CF) treatment improved J. Vet. Med. Sci. lipid metabolism in dogs, and to clarify whether its efficacy is influenced by canine characteristics. 80(6): 945–949, 2018 We collected medical records of 306 dogs and performed epidemiological analyses. Lipid values of all lipoproteins were significantly decreased by CF medication, especially VLDL triglyceride doi: 10.1292/jvms.17-0703 (TG) concentration (mean reduction rate=54.82%). However, 17.65% of dogs showed drug refractoriness in relation to TG level, and Toy Poodles had a lower CF response than other breeds (OR=5.36, 95% CI=2.07–13.90). Therefore, our study suggests that genetic factors may have an Received: 22 December 2017 effect on CF response, so genetic studies on lipid metabolism-related genes might be conducted Accepted: 9 March 2018 to identify variations in CF efficacy. Published online in J-STAGE: KEY WORDS: clinofibrate, descriptive epidemiology, drug response, dyslipidemia, Toy Poodle 26 March 2018 High serum cholesterol (Cho) and triglyceride (TG) concentrations in dogs are caused by various factors such as lack of exercise, high fat diets, obesity, neutralization, age, diseases and breed [6, 21, 24]. -

N-Acetyl Galactosamine-Conjugated Antisense Drug to APOC3 Mrna, Triglycerides and Atherogenic Lipoprotein Levels
European Heart Journal (2019) 40, 2785–2796 CLINICAL RESEARCH doi:10.1093/eurheartj/ehz209 Lipids N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels Veronica J. Alexander1, Shuting Xia1, Eunju Hurh2, Steven G. Hughes1, Louis O’Dea2, Richard S. Geary1, Joseph L. Witztum3, and Sotirios Tsimikas1,4* 1Ionis Pharmaceuticals, Inc., 2855 Gazelle Ct, Carlsbad, CA 92010, USA; 2Akcea Therapeutics, 22 Boston Wharf Road, 9th Floor, Boston, MA 02210, USA; 3Division of Endocrinology and Metabolism, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0682, USA; and 4Division of Cardiovascular Medicine, Sulpizio Cardiovascular Center, Vascular Medicine Program, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0682, USA Received 15 January 2019; revised 8 March 2019; editorial decision 25 March 2019; accepted 4 April 2019; online publish-ahead-of-print 24 April 2019 See page 2797 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz321) Aims Elevated apolipoprotein C-III (apoC-III) levels are associated with hypertriglyceridaemia and coronary heart disease. AKCEA-APOCIII-LRx is an N-acetyl galactosamine-conjugated antisense oligonucleotide targeted to the liver that selectively inhibits apoC-III protein synthesis. ................................................................................................................................................................................................... Methods The safety, tolerability, and efficacy of AKCEA-APOCIII-LRx was assessed in a double-blind, placebo-controlled, and results dose-escalation Phase 1/2a study in healthy volunteers (ages 18–65) with triglyceride levels >_90 or >_200 mg/dL. Single-dose cohorts were treated with 10, 30, 60, 90, and 120 mg subcutaneously (sc) and multiple-dose cohorts were treated with 15 and 30 mg weekly sc for 6 weeks or 60 mg every 4 weeks sc for 3 months. -

Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats
Showa Univ. J. Med. Sci. 7(2), 173•`182, December 1995 Original Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats Hideyukl KURISHIMA,Sadao NAKAYAMA,Minoru FURUYA and Katsuji OGUCHI Abstract: The effects of the clofibrate derivatives fenofibrate (FF), bezafibrate (BF), and clinofibrate (CF), on hyperlipidemia induced by a cholesterol-free, high-fructose diet (HFD) in rats were investigated. Feeding of HFD for 2 weeks increased the high-density lipoprotein subfraction (HDL1) and decreased the low-density lipoprotein (LDL) fraction. The levels of total cholesterol (TC), free cholesterol, triglyceride (TG), and phospholipid in serum were increased by HFD feeding. Administration of CF inhibited the increase in HDL1 content. All three agents inhibited the decrease in LDL level. Both BF and CF decreased VLDL level. Administration of FF, BF, or CF inhibited the increases of serum lipids, especially that of TC and TG. The inhibitory effects of CF on HFD- induced increases in HDL1, TC, and TG were greater than those of FF and BF. These results demonstrate that FF, BF, and CF improve the intrinsic hyper- lipidemia induced by HFD feeding in rats. Key words: fenofibrate, bezafibrate, clinofibrate, fructose-induced hyperlipide- mia, lipoprotein. Introduction Clofibrate is one of the most effective antihypertriglycedemic agents currently available. However, because of its adverse effects, such as hepatomegaly1, several derivatives, such as clinofibrate (CF) and bezafibrate (BF) have been developed which are more effective and have fewer adverse effects. For example, it has been shown that the hypolipidemic effect of CF is greater than that of clofibrate while its tendency to produce hepatomegaly is less1. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Statin-Induced Myopathy: Translational Studies from Preclinical to Clinical Evidence
International Journal of Molecular Sciences Review Statin-Induced Myopathy: Translational Studies from Preclinical to Clinical Evidence Giulia Maria Camerino 1, Nancy Tarantino 1 , Ileana Canfora 1 , Michela De Bellis 1, Olimpia Musumeci 2 and Sabata Pierno 1,* 1 Section of Pharmacology, Department of Pharmacy and Drug Sciences, University of Bari “Aldo Moro”, 70125 Bari, Italy; [email protected] (G.M.C.); [email protected] (N.T.); [email protected] (I.C.); [email protected] (M.D.B.) 2 Unit of Neurology and Neuromuscular Disorders, Department of Clinical and Experimental Medicine, University of Messina, 98122 Messina, Italy; [email protected] * Correspondence: [email protected] Abstract: Statins are the most prescribed and effective drugs to treat cardiovascular diseases (CVD). Nevertheless, these drugs can be responsible for skeletal muscle toxicity which leads to reduced compliance. The discontinuation of therapy increases the incidence of CVD. Thus, it is essential to assess the risk. In fact, many studies have been performed at preclinical and clinical level to investigate pathophysiological mechanisms and clinical implications of statin myotoxicity. Conse- quently, new toxicological aspects and new biomarkers have arisen. Indeed, these drugs may affect gene transcription and ion transport and contribute to muscle function impairment. Identifying a marker of toxicity is important to prevent or to cure statin induced myopathy while assuring the right therapy for hypercholesterolemia and counteracting CVD. In this review we focused on the mechanisms of muscle damage discovered in preclinical and clinical studies and highlighted the Citation: Camerino, G.M.; Tarantino, pathological situations in which statin therapy should be avoided. -

Clofibrate Causes an Upregulation of PPAR- Target Genes but Does Not
tapraid4/zh6-areg/zh6-areg/zh600707/zh65828d07a xppws S� 1 4/20/07 9: 48 MS: R-00603-2006 Ini: 07/rgh/dh A " #h!si$l %egul Integr &$ ' #h!si$l 2%&' R000 (R000) 200!$ 3. Originalarbeiten *irst publis#ed "arc# + 5) 200! doi' + 0$ + + 52,a-pregu$ 0060&$ 2006$ Clofibrate causes an upregulation of PPAR-� target genes but does not alter AQ: 1 expression of SREBP target genes in liver and adipose tissue of pigs Sebastian Luci, Beatrice Giemsa, Holger Kluge, and Klaus Eder Institut fu¨r Agrar- und Erna¨hrungswissenschaften, Martin-Luther-Universita¨t Halle-Wittenberg, Halle (Saale), Ger an! Submitted 25 August 2006 accepted in final form ! "arc# 200! AQ: 2 Luci S, Giemsa B, Kluge H, Eder K. Clofibrate causes an usuall0 increased 3#en baseline concentrations are lo3 1?62$ upregulation of PPAR-� target genes but does not alter expression of Effects of PPAR-� activation #ave been mostl0 studied in SREBP target genes in liver and adipose tissue of pigs$ A " #h!si$l rodents) 3#ic# ex#ibit a strong expression of PPAR-� in liver %egul Integr &$ ' #h!si$l 2%&' R000 (R000) 200!$ *irst publis#ed and s#o3 peroxisome proliferation in t#e liver in response to "arc# + 5) 200! doi' + 0$ + + 52,a-pregu$ 0060&$ 2006$ ./#is stud0 inves- PPAR-� activation 1&62$ Expression of PPAR-� and sensitivit0 tigated t#e effect of clofibrate treatment on expression of target genes of peroxisome proliferator-activated receptor 1PPAR2-� and various to peroxisomal induction b0 PPAR-� agonists) #o3ever) var0 genes of t#e lipid metabolism in liver and adipose tissue of pigs$ An greatl0