Limnological Aspects of the Uvs-Nuur-Basin in Northwest Mongolia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
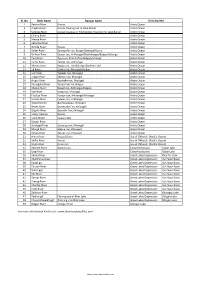
List of Rivers of Mongolia
Sl. No River Name Russian Name Draining Into 1 Yenisei River Russia Arctic Ocean 2 Angara River Russia, flowing out of Lake Baikal Arctic Ocean 3 Selenge River Сэлэнгэ мөрөн in Sükhbaatar, flowing into Lake Baikal Arctic Ocean 4 Chikoy River Arctic Ocean 5 Menza River Arctic Ocean 6 Katantsa River Arctic Ocean 7 Dzhida River Russia Arctic Ocean 8 Zelter River Зэлтэрийн гол, Bulgan/Selenge/Russia Arctic Ocean 9 Orkhon River Орхон гол, Arkhangai/Övörkhangai/Bulgan/Selenge Arctic Ocean 10 Tuul River Туул гол, Khentii/Töv/Bulgan/Selenge Arctic Ocean 11 Tamir River Тамир гол, Arkhangai Arctic Ocean 12 Kharaa River Хараа гол, Töv/Selenge/Darkhan-Uul Arctic Ocean 13 Eg River Эгийн гол, Khövsgöl/Bulgan Arctic Ocean 14 Üür River Үүрийн гол, Khövsgöl Arctic Ocean 15 Uilgan River Уйлган гол, Khövsgöl Arctic Ocean 16 Arigiin River Аригийн гол, Khövsgöl Arctic Ocean 17 Tarvagatai River Тарвагтай гол, Bulgan Arctic Ocean 18 Khanui River Хануй гол, Arkhangai/Bulgan Arctic Ocean 19 Ider River Идэр гол, Khövsgöl Arctic Ocean 20 Chuluut River Чулуут гол, Arkhangai/Khövsgöl Arctic Ocean 21 Suman River Суман гол, Arkhangai Arctic Ocean 22 Delgermörön Дэлгэрмөрөн, Khövsgöl Arctic Ocean 23 Beltes River Бэлтэсийн Гол, Khövsgöl Arctic Ocean 24 Bügsiin River Бүгсийн Гол, Khövsgöl Arctic Ocean 25 Lesser Yenisei Russia Arctic Ocean 26 Kyzyl-Khem Кызыл-Хем Arctic Ocean 27 Büsein River Arctic Ocean 28 Shishged River Шишгэд гол, Khövsgöl Arctic Ocean 29 Sharga River Шарга гол, Khövsgöl Arctic Ocean 30 Tengis River Тэнгис гол, Khövsgöl Arctic Ocean 31 Amur River Russia/China -

Genetic Variability and Divergence of Neutrodiaptomus Tumidus Kiefer 1937
Young et al. Zoological Studies 2014, 53:22 http://www.zoologicalstudies.com/content/53/1/22 RESEARCH ARTICLE Open Access Genetic variability and divergence of Neutrodiaptomus tumidus Kiefer 1937 (Copepoda: Calonida) among 10 subpopulations in the high mountain range of Taiwan and their phylogeographical relationships indicated by mtDNA COI gene Shuh-Sen Young1*, Yan-Ying Lee1 and Min-Yun Liu2 Abstract Background: In the mountain area of Taiwan, we investigated 10 subpopulations of Neutrodiaptomus tumidus Kiefer 1937 living in isolated alpine ponds or lakes. We used mitochondrial DNA cytochrome C oxidase subunit I (COI) sequence as molecular marker to investigate the population genetic structure and their phylogeographical relationships. Results: We obtained 179 sequences from 10 subpopulations and found 94 haplotypes. Nucleotide composition was AT-rich. Haplotype diversity (Hd) and nucleotide diversity (π) indicated significant genetic differences between subpopulations (Hd = 0.131 ~ 0.990; π = 0.0002 ~ 0.0084); genetic differentiation index (Fst) and gene flow index (Nm) also exhibited significant genetic diversification between subpopulations (Fst = 0.334 ~ 0.997; Nm =0~1).Using Tajima’s D and Fu and Li’s D*andF* as neutrality tests, we found that the nucleotide variation within the population was consistent with the neutral theory except in the JiaLuoHu subpopulation. The JiaLuoHu subpopulation significantly deviated from the neutral theory and was speculated to have experienced a bottleneck effect. According to the phylogenetic tree, these alpine lake subpopulations could be divided into four phylogroups (northern region, Xueshan group, central region, and southwestern region). Xueshan group contains one subpopulation, DuRongTan, which is a unique group relative to other groups. -

Confirmed Soc Reports List 2015-2016
Confirmed State of Conservation Reports for natural and mixed World Heritage sites 2015 - 2016 Nr Region Country Site Natural or Additional information mixed site 1 LAC Argentina Iguazu National Park Natural 2 APA Australia Tasmanian Wilderness Mixed 3 EURNA Belarus / Poland Bialowieza Forest Natural 4 LAC Belize Belize Barrier Reef Reserve System Natural World Heritage in Danger 5 AFR Botswana Okavango Delta Natural 6 LAC Brazil Iguaçu National Park Natural 7 LAC Brazil Cerrado Protected Areas: Chapada dos Veadeiros and Natural Emas National Parks 8 EURNA Bulgaria Pirin National Park Natural 9 AFR Cameroon Dja Faunal Reserve Natural 10 EURNA Canada Gros Morne National Park Natural 11 AFR Central African Republic Manovo-Gounda St Floris National Park Natural World Heritage in Danger 12 LAC Costa Rica / Panama Talamanca Range-La Amistad Reserves / La Amistad Natural National Park 13 AFR Côte d'Ivoire Comoé National Park Natural World Heritage in Danger 14 AFR Côte d'Ivoire / Guinea Mount Nimba Strict Nature Reserve Natural World Heritage in Danger 15 AFR Democratic Republic of the Congo Garamba National Park Natural World Heritage in Danger 16 AFR Democratic Republic of the Congo Kahuzi-Biega National Park Natural World Heritage in Danger 17 AFR Democratic Republic of the Congo Okapi Wildlife Reserve Natural World Heritage in Danger 18 AFR Democratic Republic of the Congo Salonga National Park Natural World Heritage in Danger 19 AFR Democratic Republic of the Congo Virunga National Park Natural World Heritage in Danger 20 AFR Democratic -

CHIRONOMUS Newsletter on Chironomidae Research
CHIRONOMUS Newsletter on Chironomidae Research No. 25 ISSN 0172-1941 (printed) 1891-5426 (online) November 2012 CONTENTS Editorial: Inventories - What are they good for? 3 Dr. William P. Coffman: Celebrating 50 years of research on Chironomidae 4 Dear Sepp! 9 Dr. Marta Margreiter-Kownacka 14 Current Research Sharma, S. et al. Chironomidae (Diptera) in the Himalayan Lakes - A study of sub- fossil assemblages in the sediments of two high altitude lakes from Nepal 15 Krosch, M. et al. Non-destructive DNA extraction from Chironomidae, including fragile pupal exuviae, extends analysable collections and enhances vouchering 22 Martin, J. Kiefferulus barbitarsis (Kieffer, 1911) and Kiefferulus tainanus (Kieffer, 1912) are distinct species 28 Short Communications An easy to make and simple designed rearing apparatus for Chironomidae 33 Some proposed emendations to larval morphology terminology 35 Chironomids in Quaternary permafrost deposits in the Siberian Arctic 39 New books, resources and announcements 43 Finnish Chironomidae 47 Chironomini indet. (Paratendipes?) from La Selva Biological Station, Costa Rica. Photo by Carlos de la Rosa. CHIRONOMUS Newsletter on Chironomidae Research Editors Torbjørn EKREM, Museum of Natural History and Archaeology, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway Peter H. LANGTON, 16, Irish Society Court, Coleraine, Co. Londonderry, Northern Ireland BT52 1GX The CHIRONOMUS Newsletter on Chironomidae Research is devoted to all aspects of chironomid research and aims to be an updated news bulletin for the Chironomidae research community. The newsletter is published yearly in October/November, is open access, and can be downloaded free from this website: http:// www.ntnu.no/ojs/index.php/chironomus. Publisher is the Museum of Natural History and Archaeology at the Norwegian University of Science and Technology in Trondheim, Norway. -

Dear Colleagues
NEW RECORDS OF CHIRONOMIDAE (DIPTERA) FROM CONTINENTAL FRANCE Joel Moubayed-Breil Applied ecology, 10 rue des Fenouils, 34070-Montpellier, France, Email: [email protected] Abstract Material recently collected in Continental France has allowed me to generate a list of 83 taxa of chironomids, including 37 new records to the fauna of France. According to published data on the chironomid fauna of France 718 chironomid species are hitherto known from the French territories. The nomenclature and taxonomy of the species listed are based on the last version of the Chironomidae data in Fauna Europaea, on recent revisions of genera and other recent publications relevant to taxonomy and nomenclature. Introduction French territories represent almost the largest Figure 1. Major biogeographic regions and subregions variety of aquatic ecosystems in Europe with of France respect to both physiographic and hydrographic aspects. According to literature on the chironomid fauna of France, some regions still are better Sites and methodology sampled then others, and the best sampled areas The identification of slide mounted specimens are: The northern and southern parts of the Alps was aided by recent taxonomic revisions and keys (regions 5a and 5b in figure 1); western, central to adults or pupal exuviae (Reiss and Säwedal and eastern parts of the Pyrenees (regions 6, 7, 8), 1981; Tuiskunen 1986; Serra-Tosio 1989; Sæther and South-Central France, including inland and 1990; Soponis 1990; Langton 1991; Sæther and coastal rivers (regions 9a and 9b). The remaining Wang 1995; Kyerematen and Sæther 2000; regions located in the North, the Middle and the Michiels and Spies 2002; Vårdal et al. -

Lake Baikal (Russian Federation) (N 754)/ Lac Baïkal (Fédération De Russie) (N754)
World Heritage 30 COM Patrimoine mondial Paris, 5 May / mai 2006 Original: English / anglais Distribution limited / limitée UNITED NATIONS EDUCATIONAL, SCIENTIFIC AND CULTURAL ORGANIZATION ORGANISATION DES NATIONS UNIES POUR L'EDUCATION, LA SCIENCE ET LA CULTURE CONVENTION CONCERNING THE PROTECTION OF THE WORLD CULTURAL AND NATURAL HERITAGE CONVENTION CONCERNANT LA PROTECTION DU PATRIMOINE MONDIAL, CULTUREL ET NATUREL WORLD HERITAGE COMMITTEE / COMITE DU PATRIMOINE MONDIAL Thirtieth session / Trentième session Vilnius, Lithuania / Vilnius, Lituanie 08-16 July 2006 / 08-16 juillet 2006 Item 7 of the Provisional Agenda: State of conservation of properties inscribed on the World Heritage List and/or on the List of World Heritage in Danger. Point 7 de l’Ordre du jour provisoire: Etat de conservation de biens inscrits sur la Liste du patrimoine mondial et/ou sur la Liste du patrimoine mondial en péril REPORT OF THE JOINT UNESCO-IUCN REACTIVE MONITORING MISSION RAPPORT DE MISSION DE SUIVI REACTIF CONJOINTE DE L’UNESCO ET DE L’IUCN Lake Baikal (Russian Federation) (N 754)/ Lac Baïkal (Fédération de Russie) (N754) 21-31 October 2005 / 21-31 octobre 2005 This mission report should be read in conjunction with Document: Ce rapport de mission doit être lu conjointement avec le document suivant: WHC-06/30.COM/7A WHC-06/30.COM/7A.Add WHC-06/30.COM/7B WHC-06/30.COM/7B.Add 1 World Heritage Centre – IUCN Joint Mission to Lake Baikal World Heritage Property MISSION REPORT Reactive Monitoring Mission to Lake Baikal Russian Federation 21 – 31 October 2005 Pedro Rosabal (IUCN) Guy Debonnet (UNESCO) 2 Executive summary Following previous World Heritage Committee’s discussions on the State of Conservation of this property and, prompted by reports that works on a new oil pipeline started in May 2004 within the boundaries of the property, the World Heritage Committee at its 29th session (Durban, South Africa) requested a new monitoring mission to the property. -

INFLUENCE of SOIL-ECOLOGICAL CONDITIONS on VEGETATION ZONATION in a WESTERN MONGOLIAN LAKE SHORE- SEMI-DESERT ECOTONE with 6 Figures, 4 Tables and 4 Photos
72 Erdkunde Band 61/2007 INFLUENCE OF SOIL-ECOLOGICAL CONDITIONS ON VEGETATION ZONATION IN A WESTERN MONGOLIAN LAKE SHORE- SEMI-DESERT ECOTONE With 6 figures, 4 tables and 4 photos ANDREA STRAUSS and UDO SCHICKHOFF Keywords: Vegetation geography, soil geography, environmental change, Central Asia, semidesert environments Keywords: Vegetationsgeographie, Bodengeographie, Umweltforschung, Zentralasien, aride Räume Zusammenfassung: Der Einfluss bodenökologischer Bedingungen auf die Vegetationszonierung in einem Seeufer-Halb- wüsten-Ökoton der westlichen Mongolei Das Becken der Großen Seen in der Westmongolei ist geprägt von ausgedehnten Wüsten-, Halbwüsten- und Steppen- arealen sowie von Seen und umgebenden einzigartigen Feuchtgebieten. Diese Feuchtgebiete sind bedeutende Elemente der Landschaftsökosysteme semi-arider und arider Räume. Andererseits ist die Kenntnis selbst grundlegender Ökosystemkompo- nenten wie Vegetation und Böden noch sehr lückenhaft. Das Ziel der vorliegenden landschaftsökologischen Studie entlang eines Seeufer-Halbwüsten-Ökotons war es, das klein- räumige Muster von Standortfaktoren entlang eines ausgeprägten Umweltgradienten sowie den Einfluss dieses Musters auf die Vegetationszonierung zu analysieren. Um die Veränderungen in dem Übergangsbereich zwischen aquatischen und terres- trischen Lebensräumen adäquat zu erfassen, wurden die Daten mit einem Transekt-Ansatz aufgenommen. Pflanzengesell- schaften wurden nach der Braun-Blanquet-Methode klassifiziert. Die Vegetations- und Bodendaten wurden einer Detrended Correspondence -

Genetic Diversity and Population Structure of Two Freshwater
Diversity 2013, 5, 796-810; doi:10.3390/d5040796 OPEN ACCESS diversity ISSN 1424-2818 www.mdpi.com/journal/diversity Article Genetic Diversity and Population Structure of Two Freshwater Copepods (Copepoda: Diaptomidae), Neodiaptomus schmackeri (Poppe and Richard, 1892) and Mongolodiaptomus birulai (Rylov, 1922) from Taiwan Shuh-Sen Young 1,*, Shu-Chuan Lin 1,2 and Min-Yun Liu 3 1 Department of Applied Science, National Hsinchu University of Education, Hsinchu 300, Taiwan 2 Hsinchu Municipal Hsinchu Elementary School, 106, Shingshiue Street, Hsinchu 300, Taiwan; E-Mail: [email protected] 3 National Applied Research Laboratories, Taiwan Ocean Research Institute, 3F, 106, Hoping Road, Section 2, Taipei 106, Taiwan; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +886-521-3132-2712; Fax: +886-525-7178. Received: 22 September 2013; in revised form: 15 November 2013 / Accepted: 18 November 2013 / Published: 22 November 2013 Abstract: We used the mitochondria DNA COI (cytochrome c oxidase subunit I) sequence as a genetic marker to analyze the population genetic structure of two species of freshwater copepods, Neodiaptomus schmackeri (Poppe and Richard, 1892) and Mongolodiaptomus birulai (Rylov, 1922) from Taiwan. Four populations with 51 individuals of N. schmackeri and five populations with 65 individuals of M. birulai were included. We compared the nucleotide sequences of a 635-bp fragment of the COI gene of N. schmackeri and a 655-bp fragment of the COI gene of M. birulai, and eight and 14 unique haplotypes were recorded, respectively. Tseng-Wen reservoir and Wu-San-Tao reservoir are linked by a channel, and the gene flow between them was unrestricted (Fst = 0.058; Nm = 4.04; Fst, population differentiation parameter; Nm, the number of succesfull migrants per generation); the gene flow between all other populations of both species was restricted (Fst = 0.4–0.99; Nm = 0–0.37). -

Climate Change
This “Mongolia Second Assessment Report on Climate Change 2014” (MARCC 2014) has been developed and published by the Ministry of Environment and Green Development of Mongolia with financial support from the GIZ programme “Biodiversity and adaptation of key forest ecosystems to climate change”, which is being implemented in Mongolia on behalf of the German Federal Ministry for Economic Cooperation and Development. Copyright © 2014, Ministry of Environment and Green Development of Mongolia Editors-in-chief: Damdin Dagvadorj Zamba Batjargal Luvsan Natsagdorj Disclaimers This publication may be reproduced in whole or in part in any form for educational or non-profit services without special permission from the copyright holder, provided acknowledgement of the source is made. The Ministry of Environment and Green Development of Mongolia would appreciate receiving a copy of any publication that uses this publication as a source. No use of this publication may be made for resale or any other commercial purpose whatsoever without prior permission in writing from the Ministry of Environment and Green Development of Mongolia. TABLE OF CONTENTS List of Figures . 3 List of Tables . .. 12 Abbreviations . 14 Units . 17 Foreword . 19 Preface . 22 1. Introduction. Batjargal Z. 27 1.1 Background information about the country . 33 1.2 Introductory information on the second assessment report-MARCC 2014 . 31 2. Climate change: observed changes and future projection . 37 2.1 Global climate change and its regional and local implications. Batjargal Z. 39 2.1.1 Observed global climate change as estimated within IPCC AR5 . 40 2.1.2 Temporary slowing down of the warming . 43 2.1.3 Driving factors of the global climate change . -

Lateral Gene Transfer of Anion-Conducting Channelrhodopsins Between Green Algae and Giant Viruses
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.15.042127; this version posted April 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 5 Lateral gene transfer of anion-conducting channelrhodopsins between green algae and giant viruses Andrey Rozenberg 1,5, Johannes Oppermann 2,5, Jonas Wietek 2,3, Rodrigo Gaston Fernandez Lahore 2, Ruth-Anne Sandaa 4, Gunnar Bratbak 4, Peter Hegemann 2,6, and Oded 10 Béjà 1,6 1Faculty of Biology, Technion - Israel Institute of Technology, Haifa 32000, Israel. 2Institute for Biology, Experimental Biophysics, Humboldt-Universität zu Berlin, Invalidenstraße 42, Berlin 10115, Germany. 3Present address: Department of Neurobiology, Weizmann 15 Institute of Science, Rehovot 7610001, Israel. 4Department of Biological Sciences, University of Bergen, N-5020 Bergen, Norway. 5These authors contributed equally: Andrey Rozenberg, Johannes Oppermann. 6These authors jointly supervised this work: Peter Hegemann, Oded Béjà. e-mail: [email protected] ; [email protected] 20 ABSTRACT Channelrhodopsins (ChRs) are algal light-gated ion channels widely used as optogenetic tools for manipulating neuronal activity 1,2. Four ChR families are currently known. Green algal 3–5 and cryptophyte 6 cation-conducting ChRs (CCRs), cryptophyte anion-conducting ChRs (ACRs) 7, and the MerMAID ChRs 8. Here we 25 report the discovery of a new family of phylogenetically distinct ChRs encoded by marine giant viruses and acquired from their unicellular green algal prasinophyte hosts. -

The Genome of Prasinoderma Coloniale Unveils the Existence of a Third Phylum Within Green Plants
Downloaded from orbit.dtu.dk on: Oct 10, 2021 The genome of Prasinoderma coloniale unveils the existence of a third phylum within green plants Li, Linzhou; Wang, Sibo; Wang, Hongli; Sahu, Sunil Kumar; Marin, Birger; Li, Haoyuan; Xu, Yan; Liang, Hongping; Li, Zhen; Cheng, Shifeng Total number of authors: 24 Published in: Nature Ecology & Evolution Link to article, DOI: 10.1038/s41559-020-1221-7 Publication date: 2020 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Li, L., Wang, S., Wang, H., Sahu, S. K., Marin, B., Li, H., Xu, Y., Liang, H., Li, Z., Cheng, S., Reder, T., Çebi, Z., Wittek, S., Petersen, M., Melkonian, B., Du, H., Yang, H., Wang, J., Wong, G. K. S., ... Liu, H. (2020). The genome of Prasinoderma coloniale unveils the existence of a third phylum within green plants. Nature Ecology & Evolution, 4, 1220-1231. https://doi.org/10.1038/s41559-020-1221-7 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Larvae of Gryporhynchid Cestodes (Cyclophyllidea) from Fish: a Review
FOLIA PARASITOLOGICA 51: 131–152, 2004 REVIEW ARTICLE Larvae of gryporhynchid cestodes (Cyclophyllidea) from fish: a review Tomáš Scholz1,2, Rodney A. Bray3, Roman Kuchta1,2, Radmila Řepová1 1Institute of Parasitology, Academy of Sciences of the Czech Republic and 2Faculty of Biological Sciences, University of South Bohemia, Branišovská 31, 370 05 České Budějovice, Czech Republic; 3Department of Zoology, The Natural History Museum, Cromwell Road, London SW7 5BD, UK Key words: Gryporhynchidae, Dilepididae, metacestodes, fish, species composition, host spectrum, distribution, review Abstract. Larvae (metacestodes) of tapeworms of the cyclophyllidean family Gryporhynchidae (previously included in the Dilepididae) occur in different internal organs of fresh- and brackish water fish (110 fish species of 27 families in 12 orders reported), which serve as the second intermediate hosts. The species composition, spectrum of fish hosts, sites of infection, and geographical distribution of gryporhynchids recorded from fish are reviewed here on the basis of literary data and examination of extensive material from helminthological collections. Metacestodes of the following genera have been found in fish: Amirthalingamia Bray, 1974 (1 species), Ascodilepis Guildal, 1960 (1), Cyclustera Fuhrmann, 1901 (4), Dendrouterina Fuhrmann, 1912 (1), Glossocercus Chandler, 1935 (3), Neogryporhynchus Baer et Bona, 1960 (1), Paradilepis Hsü, 1935 (5), Parvitaenia Burt, 1940 (2), and Valipora Linton, 1927 (3). However, most published records concern only three species, namely Neogryporhynchus cheilancristrotus (Wedl, 1855) from the intestinal lumen, Paradilepis scolecina (Rudolphi, 1819) from the liver and mesenteries, and Valipora campylancristrota (Wedl, 1855) from the gall bladder of cyprinids and other fish in the Palaearctic Region. Data on other species as well as reports from other regions are very scarce and almost no information is available from Australia, tropical Asia and South America.