(10) Patent No.: US 9517190 B2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transdermal and Transbuccal Drug Delivery
TRANSDERMAL AND TRANSBUCCAL DRUG DELIVERY: ENHANCMENT USING IONTOPHORESIS AND CHEMICAL ENHANCERS by LONSHENG HU A Dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey in partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Pharmaceutical Science written under the direction of Bozena Michniak-Kohn and approved by ________________________ ________________________ ________________________ ________________________ New Brunswick, New Jersey October, 2010 ABSTRACT OF THE DISSERTATION TRANSDERMAL AND TRANSBUCCAL DRUG DELIVERY: ENHANCMENT USING IONTOPHORESIS AND CHEMICAL ENHANCERS By LONSHENG HU Dissertation Director: Professor Bozena Michniak-Kohn Transdermal and transbuccal routes offer attractive alternatives for systemic delivery of drugs due to their distinct advantages: non-invasive, avoidance of first-pass effect, improved bioavailability and reduction of systemic side effects. However, only a few drugs have been successfully delivered into blood stream to reach therapeutic levels without causing notable skin irritation or damage. Transbuccal drug delivery systems are still at research stage. The major barriers to transdermal and transbuccal drug delivery are stratum corneum of skin and epithelium of buccal tissue. The objective of this work was to overcome these barriers to significantly enhance transdermal and transbuccal delivery of hydrophilic drugs without causing major damage to skin and buccal tissue. In this work, iontophoresis, chemical -

FORMULATION and EVALUATION of a MEDICATED NAIL LACQUER for the TREATMENT of ONYCHOMYCOSIS ASWANI V M Under the Guidance of DEPAR
FORMULATION AND EVALUATION OF A MEDICATED NAIL LACQUER FOR THE TREATMENT OF ONYCHOMYCOSIS Dissertation work submitted to The Tamilnadu Dr.M.G.R. Medical University ,Chennai In partial Fulfillment for the award of degree of MASTER OF PHARMACY IN PHARMACEUTICS Submitted by ASWANI V M Reg NO.261310753 Under the guidance of Dr. R.MANAVALAN, M.pharm, PhD Professor & Head Department of Pharmaceutics R.V.S College of pharmaceutical Sciences Sulur,Coimbatore (October 2015) DEPARTMENT OF PHARMACEUTICS R.V.S COLLEGE OF PHARMACEUTICAL SCIENCES SULUR, COIMBATORE-641 402, TAMIL NADU. Certificates Evaluation Certificate Dissertation title : FORMULATION AND EVALUATION OF A MEDICATED NAIL LACQUER FOR THE TREATMENT OF ONYCHOMYCOSIS Name of the Candidate : ASWANI V M Course of study : Master of Pharmacy in Pharmaceutics, Institution Name : R V S College of Pharmaceutical Sciences, Sulur, Coimbatore- 641 402 INTERNAL EXAMINER EXTERNAL EXAMINER PLACE: Coimbatoore DATE: Certificate This is to certify that the dissertation work entitled “FORMULATION AND EVALUATION OF A MEDICATED NAIL LACQUER FOR THE TREATMENT OF ONYCHOMYCOSIS “ is a bonafide work done by Aswani V M, RVS college of Pharmaceutical Sciences, Sulur, Coimbatore, for the partial fulfillment of the University rules and regulations for the award of Master of Pharmacy in Pharmaceutics under my guidance and supervision during the academic year 2015-16. Dr.R. MANAVALAN, M.pharm, PhD Professor and Head Department of Pharmaceutics R.V.S College of pharmaceutical Sciences Sulur, Coimbatore-641 402 Dr.R.VENKATANARAYANAN,M.Pharm,PhD -

Public Assessment Report Scientific Discussion Alprostadil Recordati 2
C B G M E B Public Assessment Report Scientific discussion Alprostadil Recordati 2 mg/g and 3 mg/g, cream (Alprostadil) NL/H/3303/001-002/DC Date: 21 December 2017 This module reflects the scientific discussion for the approval of Alprostadil Recordati 2 mg/g and 3 mg/g, cream. The procedure was finalised on 6 August 2015. For information on changes after this date please refer to the ‘steps taken after finalisation’ at the end of this PAR. C B G E B M List of abbreviations AE Adverse Event ANCOVA Analysis of Covariance ASMF Active Substance Master File AUC Area Under the Curve CEP Certificate of Suitability to the monographs of the European Pharmacopoeia CHMP Committee for Medicinal Products for Human Use CMD(h) Coordination group for Mutual recognition and Decentralised procedure for human medicinal products CMS Concerned Member State DDAIP Dodecyl-2-N,N-dimethylaminopropionate ED Erectile Dysfunction EDMF European Drug Master File EDQM European Directorate for the Quality of Medicines EEA European Economic Area EF Erectile Function ERA Environmental Risk Assessment EU European Union FDA Food and Drug Administration GCP Good Clinical Practice GLP Good Laboratory Practice HCl Hydrochloride ICH International Conference of Harmonisation IIEF International Index of Erectile Function IRB Institutional Review Board LADA Lauric Acid Diethanolamine LOGkow Logarithm of octanol/water partition coefficient LOQ Limit of Quantification LS Least Square MCID Minimal Clinically Important Difference MEB Medicines Evaluation Board in the Netherlands MAH Marketing Authorisation Holder PECsurfacewater Predicted Environmental Concentration in surface water Ph.Eur. European Pharmacopoeia PL Package Leaflet PSMF Pharmacovigilance System Master File PSUR Periodic Safety Update Report RH Relative Humidity RMP Risk Management Plan SAE Serious Adverse Event SD Standard Deviation SEP Sexual Encounter Profile SmPC Summary of Product Characteristics TSE Transmissible Spongiform Encephalopathy UK United Kingdom US United States 2/20 C B G E B M I. -

Insights on Antifungal Drug - Miconazole Nitrate
Human Journals Review Article July 2020 Vol.:18, Issue:4 © All rights are reserved by ABHIRAMI VENKATACHALAM et al. Insights on Antifungal Drug - Miconazole Nitrate Keywords: miconazole; systematic review; antifungal agents, dermatophytoses ABSTRACT ABHIRAMI VENKATACHALAM*, HARINI Miconazole nitrate, a synthetic derivative of imidazole, is an CHOWDARY VADLAMUDI antifungal agent used in the local treatment of vaginal, nail and 1*Acharya & BM Reddy College of Pharmacy, skin infections due to dermatophytes. Miconazole has been Soldevanahalli, Bengaluru – 560107. India. equally effective in both Candida and dermatophyte skin infections. However, it has been used effectively in chronic skin Submission: 26 June 2020 infections that have not satisfactorily reacted to other agents. It Accepted: 02 July 2020 is promising to have initial oral and intravenous miconazole Published: 30 July 2020 therapy in systemic candidiasis. Miconazole preparations are well tolerated and accepted. A greater understanding of the chemistry and specific mechanism of miconazole is critical to improve strategies and to develop better therapeutics. The purpose of this review is to enlighten the chemistry, pharmacology and the various methods used to enhance the www.ijppr.humanjournals.com solubility of miconazole. This review also includes the physicochemical properties, a brief about pharmacokinetic profile, disadvantages, drug interaction, uses and administration of miconazole nitrate. An assortment of methods has been developed in recent years that can improve its bioavailability. This article significantly reviews the latest published literature on diverse techniques for enhancing the bioavailability of miconazole. www.ijppr.humanjournals.com INTRODUCTION Miconazole is a synthetic antifungal imidazole that has been used effectively and safely in the treatment of superficial fungal infections for almost 40 years1. -

View Annual Report
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 10-K (Mark One) x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2010 OR ¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from ______ to Commission file number 0-22245 APRICUS BIOSCIENCES, INC. (Exact Name of Registrant as Specified in Its Charter) Nevada 87-0449967 (State or Other Jurisdiction of Incorporation or (I.R.S. Employer Organization) Identification No.) 6330 Nancy Ridge Drive, Suite 103, San Diego, CA 92121 (Address of Principal Executive Offices) (Zip Code) (858) 222-8041 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of Each Class Name of Exchange on Which Registered Common Stock, par value $.001 The NASDAQ Capital Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

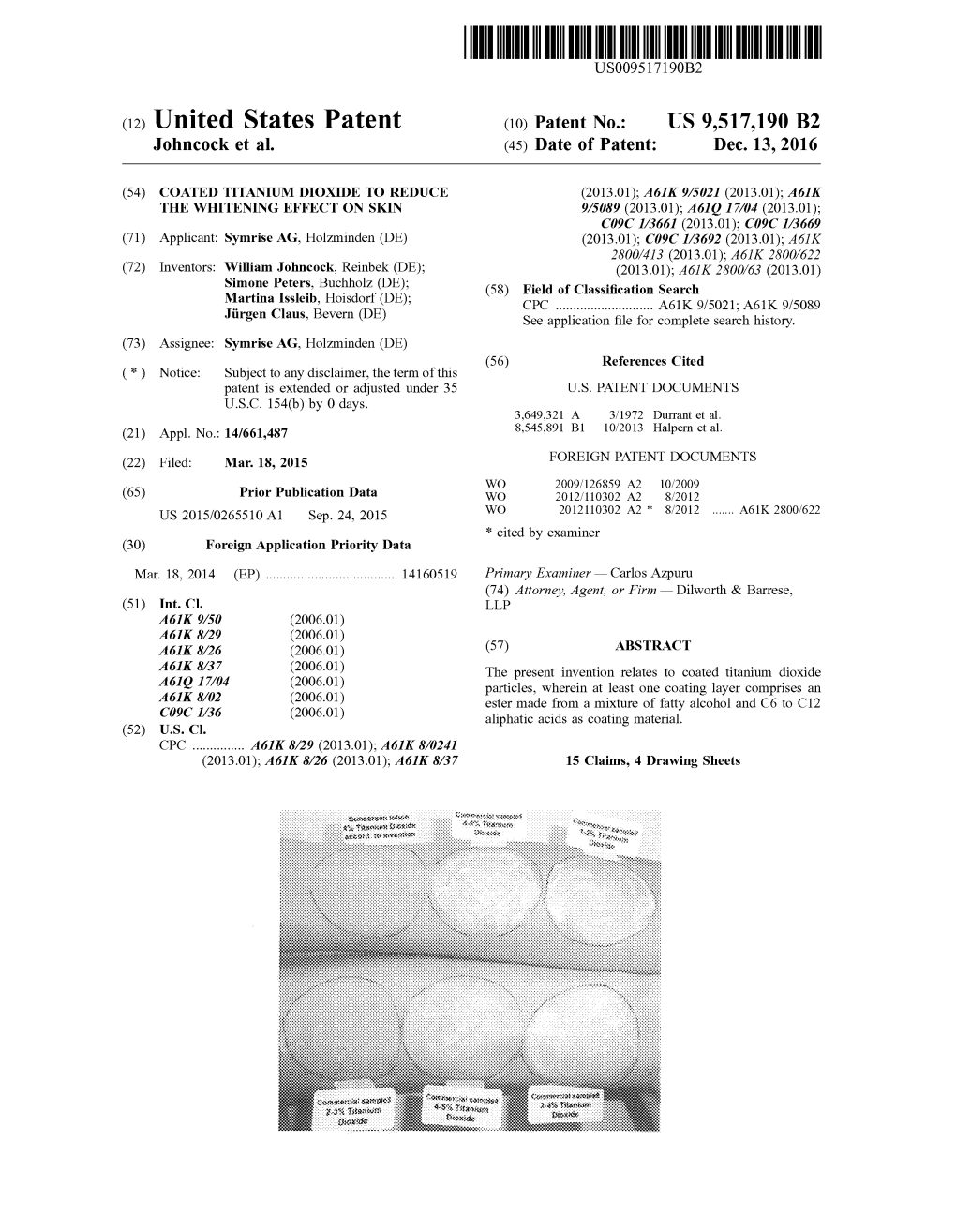
Coated Titanium Dioxide to Reduce Whitening Effect on Skin
(19) TZZ ___T (11) EP 2 921 157 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 23.09.2015 Bulletin 2015/39 A61K 8/26 (2006.01) A61K 8/29 (2006.01) A61K 8/37 (2006.01) A61Q 17/04 (2006.01) (2006.01) (2006.01) (21) Application number: 14160519.6 A61K 8/02 C09C 1/36 (22) Date of filing: 18.03.2014 (84) Designated Contracting States: • Peters, Simone AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 21244 Buchholz (DE) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • Issleib, Martina PL PT RO RS SE SI SK SM TR 22955 Hoisdorf (DE) Designated Extension States: • Claus, Jürgen BA ME 37639 Bevern (DE) (71) Applicant: Symrise AG (74) Representative: Fabry, Bernd 37603 Holzminden (DE) IP2 Patentanwalts GmbH Schlossstrasse 523 (72) Inventors: 41238 Mönchengladbach (DE) • Johncock, William 21465 Reinbek (DE) (54) Coated titanium dioxide to reduce whitening effect on skin (57) The present invention relates to coated titanium dioxide particles, wherein at least one coating layer comprises an ester made from a mixture of fatty alcohol and C6 to C12 aliphatic acids as coating material. EP 2 921 157 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 921 157 A1 Description FIELD OF INVENTION 5 [0001] The present invention belongs to the area of cosmetic and pharmaceutical preparations, especially dermato- logical preparations and refers to the protection of the human skin and human hair against the harmful effects of ultraviolet (UV) radiation. The cosmetic and pharmaceutical preparations of the invention comprise a specially coated titanium dioxide which reduces the residual whitening effect, in particular on skin after application and improves the skin feel of the finished formulation. -

Australian Public Assessment Report for Alprostadil
Australian Public Assessment Report for alprostadil Proprietary Product Name: Proshaeos Sponsor: Commercial Eyes Pty Ltd June 2016 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) • The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health and is responsible for regulating medicines and medical devices. • The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance) when necessary. • The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. • The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. • To report a problem with a medicine or medical device, please see the information on the TGA website <https://www.tga.gov.au>. About AusPARs • An Australian Public Assessment Report (AusPAR) provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission. • AusPARs are prepared and published by the TGA. • An AusPAR is prepared for submissions that relate to new chemical entities, generic medicines, major variations and extensions of indications. • An AusPAR is a static document; it provides information that relates to a submission at a particular point in time. • A new AusPAR will be developed to reflect changes to indications and/or major variations to a prescription medicine subject to evaluation by the TGA. -

Unnatural Β-Amino Acid Derivatives As Potential Transdermal Drug Delivery Systems
Unnatural β-amino acid derivatives as potential transdermal drug delivery systems Rui Filipe Jesus Pereira Mestrado em Química Departamento de Química e Bioquímica 2019/2020 Orientador Maria Luísa Cardoso do Vale, Professora Auxiliar, FCUP Coorientador Maria de La Salette Reis, Professora Catedrática, FFUP Todas as correções determinadas pelo júri, e só essas, foram efetuadas. O Presidente do Júri, Porto, ______/______/_________ FCUP III Unnatural β-amino acid derivatives as potential transdermal drug delivery systems Agradecimentos Tem sido um privilégio trabalhar neste último ano no laboratório 2.24. do DQB e ter a possibilidade de completar a minha tese de mestrado, apesar das adversidades que este ano trouxe a todos nós. Gostaria de agradecer a todas as pessoas que tornaram isto possível. Primeiro, gostaria de agradecer à minha orientadora Profª. Luísa do Vale, pela oportunidade de trabalhar num projeto inteiramente novo dentro do seu grupo de investigação, pela confiança, apoio e orientação ao longo do ano. Uma palavra de apreço e gratidão para a Dr. Cidália Pereira pois foi ela que propôs o projeto no grupo e ajudou-me nos meus primeiros passos no projeto. Sem ela não teria conseguido chegar onde cheguei. Agradeço também à Dr. Sandra Silva pelo apoio contínuo ao longo do ano, pela sua experiência e conhecimento que me ajudaram, especialmente no estudo das propriedades físico-químicas dos compostos. Agradeço ao Prof. Eduardo Marques por ter disponibilizado o seu laboratório para a realização desses mesmos estudos, e pela orientação e contribuição na análise dos resultados. Um especial agradecimento para a minha coorientadora Profª. Salette Reis (FFUP) e à Dr.