Economics of Networked Information Services
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mysore University Library LIST of JOURNALS
Mysore University Library LIST OF JOURNALS SUBJECT: CHEMISTRY PRINT JOURNALS SUBSCRIBED Journal Name Current Science Indian Chemical Society Indian Journal of Chemical Technology Indian Journal of Chemistry Section - A Indian Journal of Chemistry Section - B Indian Journal of Heterocyclic Chemistry Journal of Applied Chemistry Journal of Applied Geochemistry Journal of Chemical Science Journal of Chemistry and Chemical Sciences Research Journal of Chemistry and Environment E-JOURNALS: UGC-INFONET & MUL PUBLISHERS/ E-JOURNALS URL AGGREGATOR Accounts of Chemical Research American Chemical Society http://pubs.acs.org/journals/achre4/index.html Acs chemical biology American Chemical Society http://pubs.acs.org/journals/acbcct/index.html Acta biomaterialia ScienceDirect http://www.sciencedirect.com/science/journal/17427061 Acta crystallographicasection a Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-5724 Acta crystallographica section b Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-5740 Acta crystallographica section c Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-5759 Acta crystallographica section d Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1399-0047 Acta crystallographica section e Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-5368 (electronic) Acta crystallographica section f Blackwell - Wiley http://www.onlinelibrary.wiley.com/journal/10.1111/(ISSN)1744-3091 (electronic) -

New Journal and Database Subscriptions – 2012 -2013
NEW JOURNAL AND DATABASE SUBSCRIPTIONS – 2012 -2013 New Journals Afterall: A Journal of Art, Context and Enquiry American Biology Teacher American Journal of Bioethics American Political Thought Annals of Tourism Research Art Documentation Biodiversity and Conservation Biomaterials Science BioScience Boom: A Journal of California California Archaeology California Management Review Catalysis Science & Technology Chemical Hazards in Industry China Journal Classical Antiquity Classical Philology Crime and Justice Critical Review of International Social and Political Philosophy Education in Chemistry Educational Technology Research Development Elephant Ethics Federal Sentencing Reporter Food & Function Frankie Gastronomica: The Journal of Food and Culture Haaretz Historical Studies in the Natural Sciences HOPOS: The Journal of the International Society for the History of Philosophy of Science Huntington Library Quarterly Indian Country Today Indonesia Journal Information, Communication & Society Innovation Policy and the Economy Integrative Biology Issues in Environmental Science and Technology Journal of Applied Remote Sensing Journal of Digital Media Management Journal of Empirical Research on Human Research Ethics Journal of Environmental Studies and Sciences Journal of Human Capital Journal of Labor Economics Journal of Leisure Research Journal of Micro/Nanolithography, MEMS, and MOEMS Journal of Modern History Journal of Nanophotonics Journal of North African Studies Journal of Palestine Studies Journal of Photonics for Energy Journal -

Final-SPARC Paper Revised 2.Pdf (76.01Ko)
A study of Canadian authorship in selected SPARC Alternative journals in the early years after their introduction By John Dupuis and Leila Fernandez Steacie Science and Engineering Library, York University, Toronto, Canada Abstract The Scholarly Publishing and Academic Resources Coalition (SPARC) is an initiative of the Association of Research Libraries. In 1998 SPARC introduced the Alternative Program working with partners to launch new journals to compete with existing high- priced titles in the STM field. Currently there are 11 titles in this program listed on the website (http://www.arl.org/sparc/partner/partnerlist.html), three of which are freely accessible. This study examines the earliest adopters, Organic Letters and Evolutionary Ecology Research to determine author satisfaction with these journals. Organic Letters although originally a SPARC Alternative journal is no longer listed under this Program. A survey of Canadian authors in these journals in the first five years since inception provides insight into the reasons why they chose to publish in these journals and has definite implications for librarians. The results of these surveys are discussed in the larger framework of existing scholarly communication models. Introduction: The Scholarly Publishing and Academic Resources Coalition is well-known in the scholarly communications arena through its various partnerships and programs, its advocacy initiatives and its promotion of open access publishing. Its early development and evolution has been traced by Mary Case in Igniting Change in Scholarly Communication: SPARC, Its Past, Present, and Future, which also provides an overview of the three main publisher partnership programs SPARC Alternative, SPARC Leading Edge and SPARC Scientific Communities. -

GUT RSC Journals List
SCHEDULE A Publisher Content Section A Customer has access to the electronic versions of the following journals via an External route: Access Post - Copyright Journals E-ISSN years cancellation Owner* during Term access Analyst 1364-5528 2008-2018 2012-2018 RSC Analytical Methods 1 1759-9679 2009-2018 2012-2018 RSC Annual Reports on the Progress of Chemistry, A 1460-4760 2008-2013 2012-2013 RSC B 1460 4779 2008-2013 2012-2013 RSC C 1460-4787 2008-2013 2012-2013 RSC Biomaterials Science 1 2047-4849 2013-2018 2016-2018 RSC Catalysis Science & Technology 1 2044-4761 2011-2018 2013-2018 RSC Chemical Communications 1364-548X 2008-2018 2012-2018 RSC Chemical Science 1, 2 2041-6539 2010-2014 2012-2014 RSC Chemical Society Reviews 1460-4744 2008-2018 2012-2018 RSC Chemistry World 1749-5318 2012-2016 2012-2016 RSC CrystEngComm 1466-8033 2008-2018 2012-2018 RSC Dalton Transactions 1477-9234 2008-2018 2012-2018 RSC Education in Chemistry 1749-5326 2012-2016 2012-2016 RSC Energy & Environmental Science 1 1754-5706 2008-2018 2012-2018 RSC Environmental Science: Nano 1 2051-8161 2014-2018 2016-2018 RSC Environmental Science: Processes & Impacts including 2050-7895 2013-2018 2013-2018 RSC Journal of Environmental Monitoring (1464-0333) 2008-2012 2012 Environmental Science: Water Research & Technology 1 2053-1419 2015-2018 2017-2018 RSC Faraday Discussions 1364-5498 2008-2018 2012-2018 RSC Food & Function 1 2042-650X 2010-2018 2012-2018 RSC Green Chemistry 1463-9270 2008-2018 2012-2018 RSC Inorganic Chemistry Frontiers 1 2052-1553 2014-2018 2017-2018 -

Chemistry & Chemical Biology 2013 APR Self-Study & Documents
Department of Chemistry and Chemical Biology Self Study for Academic Program Review April, 2013 Prepared by Prof. S.E. Cabaniss, chair Table of Contents Page Executive Summary 4 I. Background A. History 5 B. Organization 8 C. External Accreditation 9 D. Previous Review 9 II. Program Goals 13 III. Curriculum 15 IV. Teaching and Learning 16 V. Students 17 VI. Faculty 21 VII. Resources and Planning 24 VIII. Facilities A. Space 25 B. Equipment 26 IX. Program Comparisons 27 X. Future Directions 31 Academic Program Review 2 Appendices A1. List of former CCB faculty A2. Handbook for Faculty Members A3. ACS Guidelines and Evaluation Procedures for Bachelor’s Degree Programs A4. Previous (2003) Graduate Review Committee report A5. UNM Mission statement A6. Academic Program Plans for Assessment of Student Learning Outcomes A7. Undergraduate degree requirements and example 4-year schedules A8. Graduate program handbook A9. CHEM 121 Parachute course A10. CHEM 122 course re-design proposal A11. Student graduation data A12. Faculty CVs A13. Staff position descriptions A14. Instrument Survey A15. CCB Annual report 2011-2012 A16. CCB Strategic Planning documents Academic Program Review 3 Executive Summary The department of Chemistry and Chemical Biology (CCB) at UNM has been in a period of transition and upheaval for several years. Historically, the department has had several overlapping missions and goals- service teaching for science and engineering majors, professional training of chemistry majors and graduate students and ambitions for a nationally-recognized research program. CCB teaches ~3% of the student credit hours taught on main campus, and at one point had over 20 tenured and tenure track faculty and ~80 graduate students. -
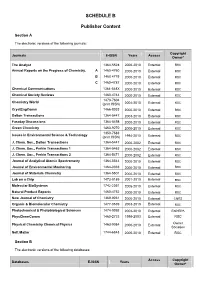
SCHEDULE B Publisher Content
SCHEDULE B Publisher Content Section A The electronic versions of the following journals: Copyright Journals E-ISSN Years Access Owner* The Analyst 1364-5528 2000-2010 External RSC Annual Reports on the Progress of Chemistry, A 1460-4760 2000-2010 External RSC B 1460 4779 2000-2010 External RSC C 1460-4787 2000-2010 External RSC Chemical Communications 1364-548X 2000-2010 External RSC Chemical Society Reviews 1460-4744 2000-2010 External RSC 1473-7604 Chemistry World (print ISSN) 2004-2010 External RSC CrystEngComm 1466-8033 2000-2010 External RSC Dalton Transactions 1364-5447 2003-2010 External RSC Faraday Discussions 1364-5498 2000-2010 External RSC Green Chemistry 1463-9270 2000-2010 External RSC 1350-7583 Issues in Environmental Science & Technology (print ISSN) 1994-2010 External RSC J. Chem. Soc., Dalton Transactions 1364-5447 2000-2002 External RSC J. Chem. Soc., Perkin Transactions 1 1364-5463 2000-2002 External RSC J. Chem. Soc., Perkin Transactions 2 1364-5471 2000-2002 External RSC Journal of Analytical Atomic Spectrometry 1364-5544 2000-2010 External RSC Journal of Environmental Monitoring 1464-0333 2000-2010 External RSC Journal of Materials Chemistry 1364-5501 2000-2010 External RSC Lab on a Chip 1473-0189 2001-2010 External RSC Molecular BioSystems 1742-2051 2005-2010 External RSC Natural Product Reports 1460-4752 2000-2010 External RSC New Journal of Chemistry 1369-9261 2000-2010 External CNRS Organic & Biomolecular Chemistry 1477-0539 2003-2010 External RSC Photochemical & Photobiological Sciences 1474-9092 2002-2010 -

Adsorption of Micropollutants on Ion Exchangers and Biosolids-Derived Biochar
Marquette University e-Publications@Marquette Dissertations, Theses, and Professional Dissertations (1934 -) Projects Micropollutant-Free Nutrient Recovery: Adsorption of Micropollutants on Ion Exchangers and Biosolids-Derived Biochar Yiran Tong Marquette University Follow this and additional works at: https://epublications.marquette.edu/dissertations_mu Part of the Environmental Engineering Commons Recommended Citation Tong, Yiran, "Micropollutant-Free Nutrient Recovery: Adsorption of Micropollutants on Ion Exchangers and Biosolids-Derived Biochar" (2018). Dissertations (1934 -). 764. https://epublications.marquette.edu/dissertations_mu/764 MICROPOLLUTANT-FREE NUTRIENT RECOVERY: ADSORPTION OF MICROPOLLUTANTS ON ION EXCHANGERS AND BIOSOLIDS-DERIVED BIOCHAR By Yiran Tong A Dissertation submitted to the Faculty of the Graduate School, Marquette University, in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Milwaukee, Wisconsin May 2018 ABSTRACT MICROPOLLUTANT-FREE NUTRIENT RECOVERY: ADSORPTION OF MICROPOLLUTANTS ON ION EXCHANGERS AND BIOSOLIDS-DERIVED BIOCHAR Yiran Tong Marquette University, 2018 The presence of excessive nutrients in treated wastewater effluent is a growing concern in terms of water quality and ecological balance. Thus, removal of nutrients is of great interest. Moreover, the removed nutrients can be recovered in forms amenable for agricultural reuse, which yields a sustainable supply of nonrenewable phosphorus that can be used to support global food production. As nutrient recovery gains -

Electronic Access Licence Agreement
ELECTRONIC ACCESS LICENCE AGREEMENT This Agreement is made the Chemistry located at ("Publisher"} and Th offices at WHEREAS (A) Publisher holds journal articles and databases in electronic form; (B) Customer wishes to license access to journal articles and/or databases; and (C) Publisher agrees to grant such licence. NOW, THEREFORE, in consideration of the mutual promises set forth herein, the parties agree as follows: 1. Definitions In thisAgreement the following terms shall have the following meanings: "Authorised Users" means current employees (including faculty, staff, and independent contractors) of the Customer and students ofthe Customer who, in either case, have been allowed access to Publlsher Content by Customer, regardless of the physical location of such persons, such access shall be unlimited and shall be by Secure Authentication so that these users may access and use Publisher Content in accordance with the terms of this Agreement. Remote access by AuthorisedUsers to Publisher Content is allowed and Walk-In Users, i.e. persons who are permitted by the Customer to access Publisher Content whilst they are on Customer's physical premises. Such access must at all times be by Secure Authentication on computer terminals within the Customer's physical premises. Walk-In Users may not be given means to access Publisher Content outside the Customer's physical premises or by any wireless network unless the network is a secure network provided by the Customer. "Commercial Use" means use which is for direct monetary reward or commercial advantage, whether by or for Customer or Authorised User, by means of the sale, resale, loan, transfer, hire or other form of exploitation of Publisher Content. -
RSC Gold Collection 2017 Excluding Journals Archive URL's Listing for 2017 Access Periodical E-ISSN Using DOI Openurl (V.0.1 Format) Openurl (V.1.0 Format) URL
RSC Gold Collection 2017 excluding Journals Archive URL's Listing for 2017 access Periodical e-ISSN Using DOI OpenURL (v.0.1 format) OpenURL (v.1.0 format) URL Analyst 1364-5528 http://dx.doi.org/10.1039/1364-5528/1876 http://xlink.rsc.org?genre=journal&eissn=1364-5528&date=1876 http://xlink.rsc.org?rft.genre=journal&rft.eissn=1364-5528&rft.date=1876 Analytical Methods 1759-9679 http://dx.doi.org/10.1039/1759-9679/2009 http://xlink.rsc.org?genre=journal&eissn=1759-9679&date=2009 http://xlink.rsc.org?rft.genre=journal&rft.eissn=1759-9679&rft.date=2009 Annual Reports on the Progress of Chemistry, Sect. A 1460-4760 http://dx.doi.org/10.1039/1460-4760/1979 http://xlink.rsc.org?genre=journal&eissn=1460-4760&date=1979 http://xlink.rsc.org?rft.genre=journal&rft.eissn=1460-4760&rft.date=1979 Annual Reports on the Progress of Chemistry, Sect. B 1460-4779 http://dx.doi.org/10.1039/1460-4779/1967 http://xlink.rsc.org?genre=journal&eissn=1460-4779&date=1967 http://xlink.rsc.org?rft.genre=journal&rft.eissn=1460-4779&rft.date=1967 Annual Reports on the Progress of Chemistry, Sect. C 1460-4787 http://dx.doi.org/10.1039/1460-4787/1979 http://xlink.rsc.org?genre=journal&eissn=1460-4787&date=1979 http://xlink.rsc.org?rft.genre=journal&rft.eissn=1460-4787&rft.date=1979 Biomaterials Science 2047-4849 http://dx.doi.org/10.1039/2047-4849/2013 http://xlink.rsc.org?genre=journal&issn=2047-4849&date=2013 http://xlink.rsc.org?rft.genre=journal&rft.issn=2047-4849&rft.date=2013 Catalysis Science & Technology 2044-4761 http://dx.doi.org/10.1039/2044-4761/2011 http://xlink.rsc.org?genre=journal&eissn=2044-4761&date=2011 -
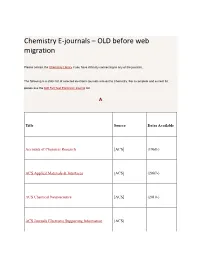
Chemistry E-Journals – OLD Before Web Migration
Chemistry E-journals – OLD before web migration Please contact the Chemistry Library if you have difficulty connecting to any of the journals. The following is a static list of selected electronic journals relevant to Chemistry. For a complete and current list please see the IUB Full Text Electronic Journal list. A Title Source Dates Available Accounts of Chemical Research [ACS] (1968-) ACS Applied Materials & Interfaces [ACS] (2007-) ACS Chemical Neuroscience [ACS] (2010-) ACS Journals Electronic Supporting Information [ACS] [Acta Chemica Acta Chemica Scandinavica (1947-1999) Scandinavica] Acta Crystallographica [IUCr] (1948-1967) Acta Crystallographica Section A: Foundations of [IUCr] (1968-) Crystallography Acta Crystallographica Section B: Structural Science [IUCr] (1968-) Acta Crystallographica Section C: Crystal Structure [IUCr] (1983-) Communications Acta Crystallographica Section D: Biological [IUCr] (1993-) Crystallography Acta Crystallographica Section E: Structure Reports [IUCr] Online (2001-) Advanced Functional Materials (continues Advanced [Wiley] Materials for Optics and Electronics) (2001-) Advanced Materials [Wiley] (1989-) Advanced Materials for Optics and Electronics (continued by Advanced Functional [Wiley] Materials) (1992-2000) Advanced Synthesis & Catalysis [Wiley] (1834-) Advances in Molecular Relaxation and Interaction [Elsevier Processes ScienceDirect] (1977-1982) Aldrichimica Acta [Sigma-Aldrich] (1968-) Amino Acids, Peptides and Proteins [RSC] (1998-2009) Analyst, The [RSC] (1876-) [Elsevier Analytica Chimica -

Dalton Transactions
Dalton Transactions View Article Online PAPER View Journal | View Issue The surprising lability of bis(2,2’:6’,2’’-terpyridine)- chromium(III) complexes† Cite this: Dalton Trans., 2014, 43, 7227 Edwin C. Constable,* Catherine E. Housecroft,* Markus Neuburger, Jonas Schönle and Jennifer A. Zampese The complex [Cr(tpy)(O3SCF3)3] (tpy = 2,2’:6’,2’’-terpyridine) is readily made from [Cr(tpy)Cl3] and is a con- venient precursor to [Cr(tpy)2][PF6]3 and to [Cr(tpy)(4’-(4-tolyl)tpy)][PF6]3 and [Cr(tpy)(5,5’’-Me2tpy)][PF6]3 (4’-(4-tolyl)tpy = 4’-(4-tolyl)-2,2’:6’,2’’-terpyridine; 5,5’’-Me2tpy = 5,5’’-dimethyl-2,2’:6’,2’’-terpyridine); these are the first examples of heteroleptic bis(tpy) chromium(III) complexes. The single crystal structures of 2{[Cr(tpy)2][PF6]3}·5MeCN, [Cr(tpy)(4’-(4-tolyl)tpy)][PF6]3·3MeCN and [Cr(tpy)(5,5’’-Me2tpy)]- 3+ [PF6]3·3MeCN have been determined. Each cation contains the expected octahedral {Cr(tpy)2} unit; in all three structures, the need to accommodate three anions per cation and the solvent molecules pre- 2+ vents the formation of a grid-like array of cations that is typical of many lattices containing {M(tpy)2} Creative Commons Attribution 3.0 Unported Licence. motifs. Three reversible electrochemical processes are observed for [Cr(tpy)(4’-(4-tolyl)tpy)][PF6]3 and 3+ [Cr(tpy)(5,5’’-Me2tpy)][PF6]3, consistent with those documented for [Cr(tpy)2] . At pH 6.36, aqueous solu- 3 tions of [Cr(tpy)2][PF6]3 are stable for at least two months. -

RSC Digital Archive and Selected Current Journals Sl.No. Title Of
RSC Digital Archive and Selected Current Journals Sl.No. Title of Journal Access URL 1 Analysts 1876 - Current http://pubs.rsc.org/en/journals/journalissues/an 2 Analytical Communications 1996 - 1999 http://pubs.rsc.org/en/journals/journalissues/ac#!issueid=ac036012&type=archive&issnprint=1359-7337 http://pubs.rsc.org/en/journals/journalissues/ap#!issueid=ap1993_30_12&type=archive&issnprint=0144- 3 Analytical Proceedings 1980 - 1993 557x Analytical Proceedings including Analytical http://pubs.rsc.org/en/journals/journalissues/ai#!issueid=ai1995_32_12&type=archive&issnprint=0144- 4 Communications 1994 - 1995 557x Annual Reports on Analytical http://pubs.rsc.org/en/journals/journalissues/aa#!issueid=aa1984_14_0&type=archive&issnprint=0306- 5 Atomic Spectroscopy 1971 - 1984 1353 Annual Reports on the Progress http://pubs.rsc.org/en/journals/journalissues/ar#!issueid=ar1966_63_0&type=archive&issnprint=0365- 6 of Chemistry 1904 - 1966 6217 Annual Reports on the Progress of Chemistry, Section A: General http://pubs.rsc.org/en/journals/journalissues/gr#!issueid=gr1972_69_0&type=archive&issnprint=0069- 7 Physical and Inorganic Chemistry 1967 - 1972 3022 Annual Reports Section "A" 8 (Inorganic Chemistry) 1979 - 2004 http://pubs.rsc.org/en/journals/journalissues/ic#!issueid=ic1979_76_0&type=current&issnprint=0260-1818 Annual Reports on the Progress of Chemistry, Section A: Physical http://pubs.rsc.org/en/journals/journalissues/pr#!issueid=pr1978_75_0&type=archive&issnprint=0308- 9 and Inorganic Chemistry 1973 - 1978 6003 Annual Reports Section