In Vitro Activities of Colistin and Sitafloxacin Combinations Against
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bangkok Anesthesia Regional Training Center
RoleRole ofof BARTCBARTC (Bangkok(Bangkok AnesthesiaAnesthesia RegionalRegional TrainingTraining Center)Center) IInn cooperationcooperation inin educationeducation andand trainingtraining inin developingdeveloping countriescountries ProfProf TharaThara TritrakarnTritrakarn DirectorDirector ofof BARTCBARTC 14th WCA, Cape Town, South Africa, 3/1/2008 Oslo Center, Norway, 12/1/2008 ShortageShortage ofof anesthesiologistsanesthesiologists AA worldwideworldwide problemsproblems MoreMore seriousserious inin developingdeveloping poorpoor countriescountries MarkedMarked variationvariation amongamong countriescountries EconomyEconomy - Most important determining factors - Three levels of wealth & health - Rich countries (per capita GNP > $ 10,000) - Medium to low (GNP $ 1,000-10,000) - Poor countries (GNP < $ 1,000) RichRich && MediumMedium countriescountries GNPGNP PeoplePeople NumberNumber PeoplePeople perper capitacapita perper ofof perper (US(US $)$) doctordoctor anesthetistsanesthetists anesthetistanesthetist USA 33,799 387 23,300 11,500 Japan 34,715 522 4,229 20,000 Singapore 22,710 667 150 26,600 Hong Kong 23,597 772 150 40,000 Australia 19,313 2170 10,000 Malaysia 3,248 1,477 250 88,000 Thailand 1,949 2,461 500 124,000 Philippines 1,048 1,016 1176 64,600 MediumMedium && PoorPoor CountriesCountries GNPGNP PeoplePeople NumberNumber PeoplePeople perper capitacapita perper ofof perper (US(US $)$) doctordoctor anesthetistsanesthetists anesthetistanesthetist Indonesia 617 6,7866,786 350 591,000591,000 Pakistan 492 2,0002,000 400 340,000340,000 -

Cover Tjs 35-4-57
ISSN 0125-6068 TheThai Journal of SURGERY Official Publication of the Royal College of Surgeons of Thailand www.surgeons.or.th/ejournal Volume 35 October-December 2014 Number 4 ORIGINAL ARTICLES 121 Comparison between Ventriculoatrial Shunt and Ventriculoperitoneal Shunt: Revision Rate and Complications Korrapakc Wangtanaphat, Porn Narischart 126 Open Surgical Management of Atherosclerotic Aortoiliac Occlusive Diseases (AIOD) Type 1 Anuwat Chantip 130 “Sawanpracharak” Connector: A Single Tube Intercostal Drainage Connector Wanchai Manakijsirisuthi 134 The Trainee’s Operative Experiences for General Surgery in Thailand Potchavit Aphinives CASE REPORT 139 Mitral and Tricuspid Valve Replacement in Uncommon Case of Situs Inversus with Dextrocardia Nuttapon Arayawudhikul, Boonsap Sakboon, Jareon Cheewinmethasiri, Angsu Chartirungsun, Benjamaporn Sripisuttrakul ABSTRACTS 143 Abstracts of the 39th Annual Scientific Congress of the Royal College of Surgeons of Thailand, 10-13 July 2014, Ambassador City Jomtien Hotel, Jomtien, Pattaya, Cholburi, Thailand (Part II) 169 Index Secretariat Office : Royal Golden Jubilee Building, 2 Soi Soonvijai, New Petchaburi Road, Huaykwang, Bangkok 10310, Thailand Tel. +66 2716 6141-3 Fax +66 2716 6144 E-mail: [email protected] www.surgeons.or.th The THAI Journal of SURGERY Official Publication of the Royal College of Surgeons of Thailand Vol. 35 October - December 2014 No. 4 Original Article Comparison between Ventriculoatrial Shunt and Ventriculoperitoneal Shunt: Revision Rate and Complications Korrapakc Wangtanaphat, MD Porn Narischart, MD Prasat Neurological Institute, Department of Medical Services, Ministry of Pubic Health, Bangkok, Thailand Abstract Background and Objective: Hydrocephalus is a common problem in neurosurgical field. In current clinical practice guidelines, ventriculoatrial shunt and ventriculoperitoneal shunt are recommended treatment options. No previous study reported differences between two procedures in term of complications and revision rates. -

September 2008 October 2008 R Dirty Pending Final 1 101 พระพุทธบาท
September 2008 October 2008 November 2008 No Site No. Hospital Name Record Status Total Cases Total Cases Total Cases Total Records r Dirty Pending Final 1 101 พระพุทธบาท สระบุรี Phraphuthabat Hospital 1129126 2 102 พญาไท ศรีราชา ชลบุรี Phyathai Sriracha Hospital 0116501 3 103 เวชศาสตรเขตรอน The Hospital for Tropical Medicine 0000000 4 104 ระยอง Rayong Hospital 155204016 5 105 รวมแพทยระยอง Ruampat Rayong 0000000 6 106 คลีนิคแพทยสุชาดา ระยอง Suchada Clinic 0000000 7 107 สงขลา Songkhla Hospital 0014202 8 108 สรรพสิทธิประสงค อุบลราชธานี Sapasittiprasong Hospital 10 21 30 120 19128 9 109 พระรามเกา Praram 9 Hospital 0000000 10 110 บําราศนราดูร นนทบุรี Bamrasnaradura Infectious Diseases Institute 0116132 11 111 ชลบุรี Chonburi Hospital 023120012 12 112 ธรรมศาสตรเฉลิมพระเกียรติ ปทุมธานี Thammasat University Hospital 0228017 13 113 เจาพระยา Chaophya Hospital 0000000 14 114 สงขลานครินทร สงขลา Songkhlanakarin Hospital 00416547 15 115 มูลนิธิโรคไต ณ โรงพยาบาลสงฆ The Kidney Foundation of Thailand 0000000 16 116 นพรัตนราชธานี Nopprat Rajathanee Hospital 036240717 17 117 พระจอมเกลา เพชรบุรี Phra Chom Klao Hospital Petburi 0000000 18 118 พระมงกุฎเกลา (กุมาร) Phramongkutklao Hospital, (Pediatrics) 0000000 19 119 สุราษฎรธานี Surat Thani Hospital 12 12 14 55 1054 20 120 รามาธิบดี Ramathibodi Hospital 0000000 21 121 วชิรพยาบาล Vajira Hospital 1112020 22 122 บานแพว สมุทรสาคร Banphaeo Hospital 1116501 23 123 สวรรคประชารักษ นครสวรรค Sawanpracharak Hospital 00312066 24 124 จุฬาลงกรณ Chulalongkorn Hospital 18 19 20 79 26 35 18 25 125 บํารุงราษฎร Bumrungrad International 0000000 26 126 ทักษิณ สุราษฎรธานี Thaksin Hospital 0000000 27 127 คายวิภาวดีรังสิต Fort Wiphavadirangsit Hospital 22215771 28 128 พระนครศรีอยุธยา Pranakornsriayudhaya Hospital 0000000 29 129 อุดรธานี Udonthani Hospital 19 22 28 112 26 57 29 30 130 ศรีนครินทร ม.ขอนแกน Srinakarin Hospital, Khon Kaen University 0000000 31 131 หนองคาย Nongkhai Hospital 0000000 32 132 ศิริราช Siriraj Hospital 1111010 Page 1 of 2 28 Jul 2008 September 2008 October 2008 November 2008 No Site No. -

Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveilla
applyparastyle “fig//caption/p[1]” parastyle “FigCapt” View metadata, citation and similar papers at core.ac.uk brought to you by CORE Open Forum Infectious Diseases provided by Apollo MAJOR ARTICLE Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveillance System Viriya Hantrakun,1, Somkid Kongyu,2 Preeyarach Klaytong,1 Sittikorn Rongsumlee,1 Nicholas P. J. Day,1,3 Sharon J. Peacock,4 Soawapak Hinjoy,2,5 and Direk Limmathurotsakul1,3,6, 1Mahidol-Oxford Tropical Medicine Research Unit (MORU), Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, 2 Epidemiology Division, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, 3 Centre for Tropical Medicine and Global Health, Nuffield Department of Clinical Medicine, Old Road Campus, University of Oxford, Oxford, United Kingdom, 4 Department of Medicine, University of Cambridge, Cambridge, United Kingdom, 5 Office of International Cooperation, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, and 6 Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand Background. National notifiable diseases surveillance system (NNDSS) data in developing countries are usually incomplete, yet the total number of fatal cases reported is commonly used in national priority-setting. Melioidosis, an infectious disease caused by Burkholderia pseudomallei, is largely underrecognized by policy-makers due to the underreporting of fatal cases via the NNDSS. Methods. Collaborating with the Epidemiology Division (ED), Ministry of Public Health (MoPH), we conducted a retrospec- tive study to determine the incidence and mortality of melioidosis cases already identified by clinical microbiology laboratories nationwide. A case of melioidosis was defined as a patient with any clinical specimen culture positive for B. -
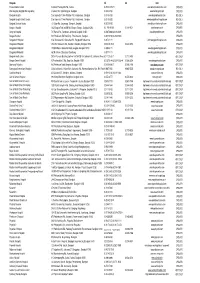
314 Provider List (For Client)@01-09-57
Bangkok tel. fax web B Care Medical Center 29 Moo 6 Phaholyothin Rd., Saimai 0-2523-3359-71 www.bcaremedicalcenter.com OPD&IPD BNH Hospital (Bangkok Nursing Home) 9 Convent Rd, Silom Bangrak , Bangkok 0-2686-2700 www.bnhhospital.com OPD&IPD Bangkok Hospital 2 Soi Soonvijai 7, New Petchburi Rd, Huaykwang , Bangkok 0-2310-3000 www.bangkokhospital.com IPD ONLY Bangkok Hospital (Heart Center) 2 Soi Soonvijai 7, New Petchburi Rd, Huaykwang , Bangkok 0-2310-3000 www.bangkokhearthospital.com IPD ONLY Bangkok Christian Hostital 124 Silom Rd., Suriyavong , Bangrak , Bangkok 0-2625-9000 www.bkkchristianhosp.th.com OPD&IPD Bangna 1 Hospital 1302 Bangna-Trad 3rd KM Rd., Bangna, Bangna, Bangkok 10260 02- 746 8630-9 02-398-9531 www.bangna.co.th OPD&IPD Bangmod Hospital 747 Rama 2 Rd., Bangmod , Jomthong, Bangkok 10150 0-2867-0606,0-2416-0049 www.bangmodhos.com OPD&IPD Bangpai Hospital 58/2 Phetkasem Rd, Pak-Klong , Phasicharoen , Bangkok 0-2457-9740, 0-2865-7948 _ OPD&IPD Bangpakok 1 Hospital 2 Soi Suksawad 25/1 Suksawad Rd., Bangpakok Ratboorana 0-2872-1111 www.bangpakokhospital.com/ OPD&IPD Bangpakok 2 Hospital 372-372/1 Eakkachai Rd., Bangbon, Bangbon, Bangkok 10150 02-899-0130-9 02-451-0357 _ OPD&IPD Bangpakok 8 Hospital 115/524 Moo. 4 Akekachai Road, Bangbon, Bangkok 10150 0-2894-4111 www.bangpakokhospital.com OPD&IPD Bangpakok 9 Hospital 362 M.4 Rama 2 Bangmod Chomthong 0-2877-1111 www.bangpakokhospital.com/ OPD&IPD Bangkok Health Clinic 2/42-43 Nusasiri Building,2nd floor Unit204-205 Soi Sukhumit 42, Sukhumvit Road ,Prakhanong,Khlongtoey02-712-0335-7,Bangkok -

Melioidosis in Animals, Thailand, 2006–2010
The Study Melioidosis in A retrospective study was performed to collect data on all animals recorded to have died of melioidosis and the Animals, Thailand, total number of livestock in Thailand during January 1, 2006–December 31, 2010. This information was obtained 2006–2010 from the Department of Livestock Development, Ministry Direk Limmathurotsakul, Suree Thammasart, of Agriculture and Cooperatives, Thailand. Information Nattachai Warrasuth, Patiporn Thapanagulsak, about animal melioidosis was derived from necropsies on Anchalee Jatapai, Vanna Pengreungrojanachai, animals that died of unknown causes which are taken to Suthatip Anun, Wacharee Joraka, the National Institute of Animal Health in central Thailand Pacharee Thongkamkoon, Piangjai Saiyen, or to 1 of 8 veterinary research and development centers Surasakdi Wongratanacheewin, throughout Thailand. Necropsy is performed principally Nicholas P.J. Day, and Sharon J. Peacock to monitor for infectious diseases that may be associated with outbreaks in farm animals. During the study period, We retrospectively estimated the incidence of culture- IHA, blood culture, and pus culture (if available) for B. proven melioidosis in animals in Thailand during 2006– pseudomallei were performed if melioidosis was suspected 2010. The highest incidence was in goats (1.63/100,000/ as the cause of death. year), followed by incidence in pigs and cattle. The Melioidosis was diagnosed as the cause of death in estimated incidence of melioidosis in humans in a given 61 animals. For 49 (80%) of these animals, diagnosis was region paralleled that of melioidosis in goats. based on a culture positive for B. pseudomallei from >1 clinical specimens; for 12 (20%) animals, cultures were elioidosis is a serious infection caused by the negative but samples were IHA positive for melioidosis Mgram-negative bacillus and biothreat organism, (based on a cutoff value of >320). -

KTB: Krung Thai Bank Public Company Limited | Annual Report
A N U L R E P O T 2 0 1 3 K r u n g h a i B k c l . TRANS FORMA TION 35 Sukhumvit Road, Klong Toey Nua Subdistrict, Wattana District, Bangkok 10110 Tel : +662 255-2222 Fax : +662 255-9391-3 KTB Call Center : 1551 Swift : KRTHTHBK http://www.ktb.co.th Annual Report 2013 K T B T r a n s f o r m a t i o n This annual report uses Green Read paper for low-eye strain and is printed with soybean ink that reduces carbon dioxide emission and has light weight for less energy consumed in delivery. Produced by : Business Risk Research Department Risk Management Sector Risk Management Group Krung Thai Bank Pcl. Designed by : Work Actually Co., Ltd. Printed by : Plan Printing Co., Ltd. Transformation…for a Sustainable Growth KTB has transformed its internal operation process and improve people capability for greater efficiency in customer services and business expansion under accurate and efficient risk management that will lead to enhanced competitiveness and readiness to make a sustainable leap together with customers, society, shareholders and stakeholders. K T B T r a n s f o r m a t i o n KTB e-Certificate ของ่าย ได้เร็ว ขอหนังสือรับรอง นิติบุคคล การประกอบธุรกิจ คนต่างด้าว สมาคมและหอการค้า 4 KTB สินเชื่อ SME เพื่อรับงานภาครัฐ หนังสือคํ้าประกันทันใจ แค่ 1 วัน Net Free Zero รับได้เลย จาก KTB netbank แบบอั้นๆ หลบไปเลย....Net Free Zero ค่าธรรมเนียม ฟรี ไม่มีอั้น ตัวจริง! มาแล้ว Annual Report 2013 Krung Thai Bank Pcl. สินเชื่อกรุงไทย 3 สบาย สินเชื่อบุคคล ที่ ให้ ชีวิต มีแต่เรื่อง สบายๆ บริการโอนเงินต่างประเทศ มุมไหนใน โลก ก็ โอนถึง ใน 1 วัน 5 สินเชื่ออเนกประสงค์ -

Acute Poisoning Surveillance in Thailand: the Current State of Affairs and a Vision for the Future
Hindawi Publishing Corporation ISRN Emergency Medicine Volume 2013, Article ID 812836, 9 pages http://dx.doi.org/10.1155/2013/812836 Review Article Acute Poisoning Surveillance in Thailand: The Current State of Affairs and a Vision for the Future Jutamas Saoraya1 and Pholaphat Charles Inboriboon2 1 Emergency Department, King Chulalongkorn Memorial Hospital, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand 2 Department of Emergency Medicine, University of Missouri-Kansas City, Kansas City, MO 64108, USA Correspondence should be addressed to Jutamas Saoraya; [email protected] Received 17 October 2013; Accepted 19 November 2013 Academic Editors: A. Eisenman, C. R. Harris, and O. Karcioglu Copyright © 2013 J. Saoraya and P. C. Inboriboon. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Acute poisoning is a major public health threat worldwide, including Thailand, a country in Southeast Asia with over 67 million inhabitants. The incidence and characteristics of poisoning in Thailand vary greatly depending on the reporting body. This systematic review aims to provide a comprehensive description of the state of poisoning in Thailand. It identifies common trends and differences in poisoning by reporting centers and regional studies. Almost half of the cases and three-fourths of the deaths involved pesticide poisonings associated with agricultural occupations. However, increasing urbanization has led to an increase in drug and household chemical poisoning. Though the majority of reported poisonings remain intentional, a trend towards unintentional poisonings in pediatric and geriatric populations should not be dismissed. -

Costs of Doing Business in Thailand 2008
Thailand Board of Investment COSTS OF DOING BUSINESS IN THAILAND 2008 CONTENTS Page Typical Costs of Starting and Operating a Business 4 Selected Costs of Living in Bangkok 6 Tax Rates and Double Taxation Agreements 12 Labor Costs 16 Transportation Costs, Including Fuel and Freight Rates 20 Utility Costs 23 Communication Costs 26 Industrial Estates and Facilities 30 Misc. Information Vehicle Sales 50 Engineering, Skilled Labor, and other related occupations in Thailand 50 Driving Distance Chart 51 List Of International School 52 Universities Offering International Programs 59 Chambers Of Commerce 61 List of Low Cost Airlines in Thailand 63 List of Hospitals 64 Hotels 66 Equipments 73 Sea Ports in Thailand 86 Airports in Thailand 88 Contact Us 90 TYPICAL COSTS OF STARTING AND OPERATING A BUSINESS 1. Visas (Government fee) (1) Baht US$ Work permit (process time 1-10 days) 3 months 750 23.01 6 months 1,500 46.01 1 year 3,000 92.02 One-year visa, 1-30 days 1,900 58.28 Re-entry visa (process time 2 days) Single entry 1,000 30.67 Multiple entry 3,800 116.56 1a. Visas (Typical fee charged by a law firm to process) (1) Work permit (new), 1-14 days 38,000 1,165.64 Visa extension, 1-30 days 28,000 858.90 Re-entry visa, 2 days 2,200 67.48 2. Registration (Government fee) (1) Company registration, 21 days 5,500 per 1 million 168.71 per 30,674.85 (275,000 maximum) (8,435.58) List 2) Alien business license, 60 days 40,000-500,000 1,226.99-15,337.42 List 3) Alien business license, 60 days 20,000-250,000 613.50-7,668.71 Factory license, 30-45 days 500-60,000 15.34-1,840.49 3. -

In Vitro Activity of Biapenem and Comparators Against Multidrug
Pharmaceutical Sciences Asia Pharm Sci Asia 2020; 47 (4), 378-386 DOI:10.29090/psa.2020.04.019.0072 Research Article In vitro activity of biapenem and comparators against multidrug-resistant and carbapenem-resistant Acinetobacter baumannii isolated from tertiary care hospitals in Thailand Jantana Houngsaitong1, Preecha Montakantikul1, Taniya Paiboonwong1, ABSTRACT 2 Mullika Chomnawang , Acinetobacter baumannii is one of nosocomial pathogen 2 Piyatip Khuntayaporn , which emerges as multidrug-resistance worldwide. Multidrug- 1* Suvatna Chulavatnatol resistant A. baumannii (MDRAB) and carbapenem-resistant A. baumannii (CRAB) are highly concerned due to limitation of 1 Department of Pharmacy, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand therapeutic options. Antibacterial activity of biapenem was explored 2 Department of Microbiology, Faculty of in order to overcome bacterial resistance. A total of 412 A. baumannii Pharmacy, Mahidol University, Bangkok, Thailand clinical isolates from 13 tertiary care hospitals in Thailand were collected. MIC values of biapenem and comparators; imipenem, meropenem, colistin, sulbactam, ciprofloxacin ceftazidime and *Corresponding author: fosfomycin sodium, were determined by broth microdilution Suvatna Chulavatnatol [email protected] method in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (2016). In total, 320 isolates (77.67%) were MDRAB and 328 isolates (79.61%) were CRAB while 58 isolates (14.07%) were colistin-resistant AB. A. baumannii showed widespread resistance to ceftazidime, ciprofloxacin KEYWORDS: and carbapenems in more than 90% of the strains; resistance to Biapenem; Multidrug-resistant A. sulbactam, fosfomycin, and colistin were 85%, 60%, and 15% Baumannii; Carbapenem-resistant respectively. By comparison among carbapenems, biapenem showed MIC of 16/32 which were at least 2 folds lower A. -

ASSOCIATED GENES, and GENOTYPE of METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS Clinical ISOLATES from NORTHERN THAILAND
SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH ANTIBIOGRAM, ANTIBIOTIC AND DISINFECTANT RESISTANCE GENES, BIOFILM-PRODUCING AND -ASSOCIATED GENES, AND GENOTYPE OF METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS CLINIcaL ISOLATES FROM NORTHERN THAILAND Rathanin Seng1, Thawatchai Kitti2, Rapee Thummeepak3, Autchasai Siriprayong3, Thitipan Phukao3, Phattaraporn Kongthai3, Thanyasiri Jindayok4, Kwanjai Ketwong5, Chalermchai Boonlao6 and Sutthirat Sitthisak3,7 1Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Bangkok; 2Faculty of Oriental Medicine, Chiang Rai College, Chiang Rai; 3Department of Microbiology and Parasitology, Faculty of Medical Science, 4Division of Clinical Pathology, Faculty of Medicine, Naresuan University, Phitsanulok; 5Sawanpracharak Hospital, Amphoe Meuang, Nakhon Sawan; 6Chiang Rai Prachanukroh Hospital, Amphoe Meuang, Chiang Rai; 7Centre of Excellence in Medical Biotechnology, Faculty of Medical Science, Naresuan University, Phitsanulok, Thailand Abstract. Methicillin-resistant Staphylococcus aureus (MRSA) causes a variety of infectious diseases in both hospital and community. The study determined preva- lence of antibiotic resistance and associated genes, biofilm-producing phenotype and associated genes, SCCmec types, and clonal subtype ST239 of MRSA clinical isolates obtained from three hospitals in northern Thailand during January 2013 to October 2015. Some 95% of MRSA isolates were multidrug resistant, with 82%, 60% and 47% harboring ermA, ermB and qacAB, respectively. Although all MRSA isolates were positive for slime (biofilm) production on Congo red agar, quan- titative measurement of biofilm generation using microtiter plate assay (MTP) indicated 60% were low biofilm producers, with prevalence of biofilm-associated genes, bab, cna, fnbA, and icaAD, ranging from 50% to 100%. MRSA SCCmec type III was predominant, but the presence of SCCmec type IV and type V (albeit at low frequency) indicated acquisition of community-acquired infection. -

Journal of Public Health Nursing Vol. 31, No. 2 May - August 2017
Journal of Public Health Nursing Vol. 31, No. 2 May - August 2017 Research page Effect of a Case Management Program for Older People with Diabetic 1 Retinopathy Patcharaporn Kerdmongkol Kwanjai Amnatsatsue Pornthip Rattanasoungthum The Effect of a Self-Management and Social Support Programfor on New Cases 4 with Type 2 Diabetes Khanitta Intaboot Surintorn Kalampakorn Panan Pichayapinyo Factors Related to Spiritual Well-Being among Caregivers of Schizophrenic Patients 10 Lerluk Mahiphun Chanudda Nabkasorn Duangjai Vatanasin Factors Influencing Depression among Adolescents in Extended Opportunity 19 Schools Prawnapa Boonprathum Pornpat Hengudomsub Duangjai Vattasin Factors Associated with Safety Behavior among Vocational Students in the 23 Vocational Program at Chitralada Vocational School Chorjit Rungsiri Plernpit Suwan-ampai Orawan Keawboonchoo Pimpan Silpasuwan Ann Jirapongsuwan Relationship of the Readiness of Community Health Nursing Practice and Mental 28 Health of Second Year Nursing Students, Kasem Bundit University Siriluk Suesat Life Assets and Factors Related to Early Smoking Stage among Male Upper 33 Primary School Students, Kalasin Sureerut Wiangkamon Pornnapa Homsin Rungrat Srisuriyawet Alcohol drinking behavior among undergraduate students in Phayao Province 39 Wichanee Jaimalai Wilaiporn Wongkeenee Kesorn Ketchu Sirirat Kosalwat Cholada Chaikoolvatana The Effects of a Teacher Development Program on Child Development among 43 Preschool Children in Child Care Centers Pradub Srimuenwai Naruemon Auemaneekul Punyarat Lapvongwatana