Regional Biodiversity Management Strategy : Case Study on the Flinders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Draft Strategic Plan for the Eyre Peninsula Natural Resources Management Region 2017 - 2026
EYRE PENINSULA NRM PLAN Draft Strategic Plan for the Eyre Peninsula Natural Resources Management Region 2017 - 2026 PAGE 1 MINISTER’S ENDORSEMENT I, Honourable Ian Hunter MLC, Minister for Sustainability, Environment and Conservation, after taking into account and in accordance with the requirements of Section 81 of the Natural Resources Management Act 2004 hereby approve the Strategic Plan of the Eyre Regional Natural Resources Management Region. n/a until adoption Honourable Ian Hunter MLC Date: Minister for Sustainability, Environment and Conservation Document control Document owner: Eyre Peninsula Natural Resources Management Board Name of document: Strategic Plan for the Eyre Peninsula Natural Resources Management Region 2017-2026 Authors: Anna Pannell, Nicole Halsey and Liam Sibly Version: 1 Last updated: Monday, 28 November, 2016 FOREWORD On behalf of the Eyre Peninsula Natural Resources Management Board (the Board), I am delighted to present our Strategic Plan for statutory consultation. The Strategic Plan is a second generation plan, building upon 2009 plan. Our vision remains - Natural resources managed to support ecological sustainability, vibrant communities and thriving enterprises in a changing climate The Strategic Plan is designed to be the “Region’s Plan”, where we have specifically included a range of interests and values in Natural Resources Management (NRM). The Board used a participatory approach to develop the plan, which allowed us to listen to and discuss with local communities, organisations and businesses about the places and issues of importance. This approach has built our shared understanding, broadened our perspectives and allowed us to capture a fair representation of the region’s interests and values. -

Released Under Foi
File 2018/15258/01 – Document 001 Applicant Name Applicant Type Summary All briefing minutes prepared for Ministers (and ministerial staff), the Premier (and staff) and/or Deputy Premier (and staff) in respect of the Riverbank precinct for the period 2010 to Vickie Chapman MP MP present Total patronage at Millswood Station, and Wayville Station (individually) for each day from 1 Corey Wingard MP October 30 November inclusive Copies of all documents held by DPTI regarding the proposal to shift a government agency to Steven Marshall MP Port Adelaide created from 2013 to present The total annual funding spent on the Recreation and Sport Traineeship Incentive Program Tim Whetstone MP and the number of students and employers utilising this program since its inception A copy of all reports or modelling for the establishment of an indoor multi‐sports facility in Tim Whetstone MP South Australia All traffic count and maintenance reports for timber hulled ferries along the River Murray in Tim Whetstone MP South Australia from 1 January 2011 to 1 June 2015 Corey Wingard MP Vision of rail car colliding with the catenary and the previous pass on the down track Rob Brokenshire MLC MP Speed limit on SE freeway during a time frame in September 2014 Request a copy of the final report/independent planning assessment undertaken into the Hills Face Zone. I believe the former Planning Minister, the Hon Paul Holloway MLC commissioned Steven Griffiths MP MP the report in 2010 All submissions and correspondence, from the 2013/14 and 2014/15 financial years -

Aboriginal Agency, Institutionalisation and Survival
2q' t '9à ABORIGINAL AGENCY, INSTITUTIONALISATION AND PEGGY BROCK B. A. (Hons) Universit¡r of Adelaide Thesis submitted for the degree of Doctor of Philosophy in History/Geography, University of Adelaide March f99f ll TAT}LE OF CONTENTS ii LIST OF TAE}LES AND MAPS iii SUMMARY iv ACKNOWLEDGEMENTS . vii ABBREVIATIONS ix C}IAPTER ONE. INTRODUCTION I CFIAPTER TWO. TI{E HISTORICAL CONTEXT IN SOUTH AUSTRALIA 32 CHAPTER THREE. POONINDIE: HOME AWAY FROM COUNTRY 46 POONINDIE: AN trSTä,TILISHED COMMUNITY AND ITS DESTRUCTION 83 KOONIBBA: REFUGE FOR TI{E PEOPLE OF THE VI/EST COAST r22 CFIAPTER SIX. KOONIBBA: INSTITUTIONAL UPHtrAVAL AND ADJUSTMENT t70 C}IAPTER SEVEN. DISPERSAL OF KOONIBBA PEOPLE AND THE END OF TI{E MISSION ERA T98 CTIAPTER EIGHT. SURVTVAL WITHOUT INSTITUTIONALISATION236 C}IAPTER NINtr. NEPABUNNA: THtr MISSION FACTOR 268 CFIAPTER TEN. AE}ORIGINAL AGENCY, INSTITUTIONALISATION AND SURVTVAL 299 BIBLIOGRAPI{Y 320 ltt TABLES AND MAPS Table I L7 Table 2 128 Poonindie location map opposite 54 Poonindie land tenure map f 876 opposite 114 Poonindie land tenure map f 896 opposite r14 Koonibba location map opposite L27 Location of Adnyamathanha campsites in relation to pastoral station homesteads opposite 252 Map of North Flinders Ranges I93O opposite 269 lv SUMMARY The institutionalisation of Aborigines on missions and government stations has dominated Aboriginal-non-Aboriginal relations. Institutionalisation of Aborigines, under the guise of assimilation and protection policies, was only abandoned in.the lg7Os. It is therefore important to understand the implications of these policies for Aborigines and Australian society in general. I investigate the affect of institutionalisation on Aborigines, questioning the assumption tl.at they were passive victims forced onto missions and government stations and kept there as virtual prisoners. -

Northern Flinders Ranges
A B Oodnadatta Track C D Innamincka E F Birdsville Track Strzelecki ROAD CONDITIONS The road surface information on this map Track should be used as a guide only. Local TRACK advice should be sought at all times. LAKES Frome With very few exceptions, the lakes STRZELECKI Arkaroola Paralana 'Mt Lyndhurst' Wilderness Hot Springs 1 shown are dry salt pans and do not Ochre Pits Sanctuary 'North Mulga' 1 indicate a permanent source of water. 'Avondale' 'Umberatana' Mt Painter PASTORAL PROPERTIES Lyndhurst The roads in this region pass through Talc Alf Echo Camp working pastoral properties. Please do Backtrack Barraranna Gorge not leave the road and enter these River 4WD properties without prior permission from Arkaroola 'Yankaninna' Ochre the landholder. Most home steads do Wall not provide tourist facilities and are 83 Wooltana shown on this map for navigational Vulkathunha - Cave purposes. Please respect the property 33 Mainwater Pound and privacy of pastoralists. 'Owieandana' 'Wooltana' Coaleld Illinawortina 'Myrtle Springs' Gammon Ranges 30 4WD TRACKS National Park Pound For more information on 4WD Tracks Gerti Johnson Nepouie please obtain a copy of the 4WD Tracks 'Leigh Monument Weetootla Gorge 2 & Repeater Towers brochure. You may Creek' Balcanoona Gorge 2 need to make an appointment and pay Ck 'Depot NEPABUNNA access fees for some tracks. Copley 45 Springs' ABORIGINAL LAND Leigh Creek Nepabunna Balcanoona Aroona Dam 'Angepena' 54 Italowie Nat. Park H.Q. Fence Arrunha Aroona Iga Warta Gorge Sanctuary 'Maynards Vulkathunha - Gammon Ranges Well' National Park Puttapa 'Wertaloona' Copper King Mine Puttapa Moro Gorge Gap HWY NANTAWARRINA Ediacara 'Warraweena' INDIGENOUS Dog Lake Conservation Lake Reserve Afghan PROTECTED AREA 39 Mon. -

On Track 2010-2011
ON TRACK Delivering NRM in the SA Arid Lands 2010-11 ON TRACK Delivering natural resources management in the SA Arid Lands 2010-11 Protecting our land, plants and animals Understanding and securing our water resources Supporting our industries and communities 1 Welcome It is with great pleasure that I introduce this first edition of On Track. Having now completed the first year the achievements of former Presiding of delivery of the South Australian Arid Member Chris Reed, previous members Lands (SAAL) Regional Natural Resources of the Board, and General Manager John Management (NRM) Plan which sets the Gavin. Almost all of the activities you will direction for natural resources management read about here were initiated through their in the region to 2020, On Track is a report efforts and the current Board is building on to our community on the progress we made their endeavours. in 2010-11 on meeting the Plan’s targets. This year was also marked by the True to the SAAL NRM Board’s platform establishment of the new Department of and the spirit of natural resources Environment and Natural Resources in July management, On Track’s focus is on 2010 which brings together staff from the community. Outback office of the former Department We showcase the variety of projects and for Environment and Heritage and the staff activities where community members are of the SAAL NRM Board. working with the Board. This new integrated service will use a We share with you the experiences of landscape approach to manage natural some of the landholders and community resources across public and private land members involved with our programs and provide a single face for environment including Ecosystem Management and natural resources services in our Understanding™, Pest Management and region. -

National Recovery Plan for the Plains Mouse Pseudomys Australis 2012
National Recovery Plan for the Plains Mouse Pseudomys australis 2012 - 1 - This plan should be cited as follows: Moseby, K. (2012) National Recovery Plan for the Plains Mouse Pseudomys australis. Department of Environment, Water and Natural Resources, South Australia. Published by the Department of Environment, Water and Natural Resources, South Australia. Adopted under the Environment Protection and Biodiversity Conservation Act 1999: [date to be supplied] ISBN : 978-0-9806503-1-0 © Department of Environment, Water and Natural Resources, South Australia. This publication is copyright. Apart from any use permitted under the copyright Act 1968, no part may be reproduced by any process without prior written permission from the Government of South Australia. Requests and inquiries regarding reproduction should be addressed to: Department of Environment, Water and Natural Resources GPO Box 1047 ADELAIDE SA 5001 Note: This recovery plan sets out the actions necessary to stop the decline of, and support the recovery of, the listed threatened species or ecological community. The Australian Government is committed to acting in accordance with the plan and to implementing the plan as it applies to Commonwealth areas. The plan has been developed with the involvement and cooperation of a broad range of stakeholders, but individual stakeholders have not necessarily committed to undertaking specific actions. The attainment of objectives and the provision of funds may be subject to budgetary and other constraints affecting the parties involved. Proposed actions may be subject to modification over the life of the plan due to changes in knowledge. Queensland disclaimer: The Australian Government, in partnership with the Queensland Department of Environment and Heritage Protection, facilitates the publication of recovery plans to detail the actions needed for the conservation of threatened native wildlife. -
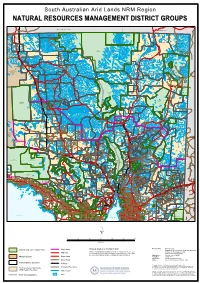
Natural Resources Management District Groups
South Australian Arid Lands NRM Region NNAATTUURRAALL RREESSOOUURRCCEESS MMAANNAAGGEEMMEENNTT DDIISSTTRRIICCTT GGRROOUUPPSS NORTHERN TERRITORY QUEENSLAND Mount Dare H.S. CROWN POINT Pandie Pandie HS AYERS SIMPSON DESERT RANGE SOUTH Tieyon H.S. CONSERVATION PARK ALTON DOWNS TIEYON WITJIRA NATIONAL PARK PANDIE PANDIE CORDILLO DOWNS HAMILTON DEROSE HILL Hamilton H.S. SIMPSON DESERT KENMORE REGIONAL RESERVE Cordillo Downs HS PARK Lambina H.S. Mount Sarah H.S. MOUNT Granite Downs H.S. SARAH Indulkana LAMBINA Todmorden H.S. MACUMBA CLIFTON HILLS GRANITE DOWNS TODMORDEN COONGIE LAKES Marla NATIONAL PARK Mintabie EVERARD PARK Welbourn Hill H.S. WELBOURN HILL Marla - Oodnadatta INNAMINCKA ANANGU COWARIE REGIONAL PITJANTJATJARAKU Oodnadatta RESERVE ABORIGINAL LAND ALLANDALE Marree - Innamincka Wintinna HS WINTINNA KALAMURINA Innamincka ARCKARINGA Algebuckinna Arckaringa HS MUNGERANIE EVELYN Mungeranie HS DOWNS GIDGEALPA THE PEAKE Moomba Evelyn Downs HS Mount Barry HS MOUNT BARRY Mulka HS NILPINNA MULKA LAKE EYRE NATIONAL MOUNT WILLOUGHBY Nilpinna HS PARK MERTY MERTY Etadunna HS STRZELECKI ELLIOT PRICE REGIONAL CONSERVATION ETADUNNA TALLARINGA PARK RESERVE CONSERVATION Mount Clarence HS PARK COOBER PEDY COMMONAGE William Creek BOLLARDS LAGOON Coober Pedy ANNA CREEK Dulkaninna HS MABEL CREEK DULKANINNA MOUNT CLARENCE Lindon HS Muloorina HS LINDON MULOORINA CLAYTON Curdimurka MURNPEOWIE INGOMAR FINNISS STUARTS CREEK SPRINGS MARREE ABORIGINAL Ingomar HS LAND CALLANNA Marree MUNDOWDNA LAKE CALLABONNA COMMONWEALTH HILL FOSSIL MCDOUAL RESERVE PEAK Mobella -

M01: Mineral Exploration Licence Applications
M01 Mineral Exploration Licence Applications 27 September 2021 Resources and Energy Group L4 11 Waymouth Street, Adelaide SA 5000 http://energymining.sa.gov.au/minerals GPO Box 320, ADELAIDE, SA 5001 http://energymining.sa.gov.au/energy_resources Phone +61 8 8463 3000 http://map.sarig.sa.gov.au Email [email protected] Earth Resources Information Sheet - M1 Printed on: 27/09/2021 M01: Mineral Exploration Licence Applications Year Lodged: 1996 File Ref. Applicant Locality Fee Zone Area (km2 ) 250K Map 1996/00118 NiCul Minerals Limited Mount Harcus area - Approx 400km 2,415 Lindsay, WNW of Marla Woodroffe 1996/00185 NiCul Minerals Limited Willugudinna Hill area - Approx 823 Everard 50km NW of Marla 1996/00260 Goldsearch Ltd Ernabella South area - Approx 519 Alberga 180km NW of Marla 1996/00262 Goldsearch Ltd Marble Hill area - Approx 80km NW 463 Abminga, of Marla Alberga 1996/00340 Goldsearch Ltd Birksgate Range area - Approx 2,198 Birksgate 380km W of Marla 1996/00341 Goldsearch Ltd Ayliffe Hill area - Approx 220km NW 1,230 Woodroffe of Marla 1996/00342 Goldsearch Ltd Musgrave Ranges area - Approx 2,136 Alberga 180km NW of Marla 1996/00534 Caytale Pty Ltd Bull Hill area - Approx 240km NW of 1,783 Woodroffe Marla Year Lodged: 1997 File Ref. Applicant Locality Fee Zone Area (km2 ) 250K Map 1997/00040 Minex (Aust) Pty Ltd Bowden Hill area - Approx 300 WNW 1,507 Woodroffe of Marla 1997/00053 Mithril Resources Limited Mt Cooperina Area - approx. 360 km 1,013 Mann WNW of Marla 1997/00055 Mithril Resources Limited Oonmooninna Hill Area - approx. -

MARREE - INNAMINCKA Natural Resources Management Group
South Australian Arid Lands NRM Region MARREE - INNAMINCKA Natural Resources Management Group NORTHERN TERRITORY QUEENSLAND SIMPSON DESERT CONSERVATION PARK Pastoral Station ALTON DOWNS MULKA PANDIE PANDIE Boundary CORDILLO DOWNS Conservation and National Parks Regional reserve/ SIMPSON DESERT Pastoral Station REGIONAL RESERVE Aboriginal Land Marree - Innamincka CLIFTON HILLS NRM Group COONGIE LAKES NATIONAL PARK INNAMINCKA REGIONAL RESERVE SA Arid Lands NRM Region Boundary INNAMINCKA Dog Fence COWARIE Major Road MACUMBA ! KALAMURINA Innamincka Minor Road / Track MUNGERANIE Railway GIDGEALPA ! Moomba Cadastral Boundary THE PEAKE Watercourse LAKE EYRE (NORTH) LAKE EYRE MULKA Mainly Dry Lake NATIONAL PARK MERTY MERTY STRZELECKI ELLIOT PRICE REGIONAL CONSERVATION RESERVE PARK ETADUNNA BOLLARDS ANNA CREEK LAGOON DULKANINNA MULOORINA LINDON LAKE BLANCHE LAKE EYRE (SOUTH) MULOORINA CLAYTON MURNPEOWIE Produced by: Resource Information, Department of Water, Curdimurka ! STRZELECKI Land and Biodiversity Conservation. REGIONAL Data source: Pastoral lease names and boundaries supplied by FINNISS MARREE RESERVE Pastoral Program, DWLBC. Cadastre and Reserves SPRINGS LAKE supplied by the Department for Environment and CALLANNA ABORIGINAL ! Marree CALLABONNA Heritage. Waterbodies, Roads and Place names LAND FOSSIL supplied by Geoscience Australia. STUARTS CREEK MUNDOWDNA Projection: MGA Zone 53. RESERVE Datum: Geocentric Datum of Australia, 1994. MOOLAWATANA MOUNT MOUNT LYNDHURST FREELING FARINA MULGARIA WITCHELINA UMBERATANA ARKAROOLA WALES SOUTH NEW -

Olympic Dam Expansion
OLYMPIC DAM EXPANSION DRAFT ENVIRONMENTAL IMPACT STATEMENT 2009 APPENDIX P CULTURAL HERITAGE ISBN 978-0-9806218-0-8 (set) ISBN 978-0-9806218-4-6 (appendices) APPENDIX P CULTURAL HERITAGE APPENDIX P1 Aboriginal cultural heritage Table P1 Aboriginal Cultural Heritage reports held by BHP Billiton AUTHOR DATE TITLE Antakirinja Incorporated Undated – circa Report to Roxby Management Services by Antakirinja Incorporated on August 1985 Matters Related To Aboriginal Interests in The Project Area at Olympic Dam Anthropos Australis February 1996 The Report of an Aboriginal Ethnographic Field Survey of Proposed Works at Olympic Dam Operations, Roxby Downs, South Australia Anthropos Australis April 1996 The Report of an Aboriginal Archaeological Field Survey of Proposed Works at Olympic Dam Operations, Roxby Downs, South Australia Anthropos Australis May 1996 Final Preliminary Advice on an Archaeological Survey of Roxby Downs Town, Eastern and Southern Subdivision, for Olympic Dam Operations, Western Mining Corporation Limited, South Australia Archae-Aus Pty Ltd July 1996 The Report of an Archaeological Field Inspection of Proposed Works Areas within Olympic Dam Operations’ Mining Lease, Roxby Downs, South Australia Archae-Aus Pty Ltd October 1996 The Report of an Aboriginal Heritage Assessment of Proposed Works Areas at Olympic Dam Operations’ Mining Lease and Village Site, Roxby Downs, South Australia (Volumes 1-2) Archae-Aus Pty Ltd April 1997 A Report of the Detailed Re-Recording of Selected Archaeological Sites within the Olympic Dam Special -
Wool Statistical Area's
Wool Statistical Area's Monday, 24 May, 2010 A ALBURY WEST 2640 N28 ANAMA 5464 S15 ARDEN VALE 5433 S05 ABBETON PARK 5417 S15 ALDAVILLA 2440 N42 ANCONA 3715 V14 ARDGLEN 2338 N20 ABBEY 6280 W18 ALDERSGATE 5070 S18 ANDAMOOKA OPALFIELDS5722 S04 ARDING 2358 N03 ABBOTSFORD 2046 N21 ALDERSYDE 6306 W11 ANDAMOOKA STATION 5720 S04 ARDINGLY 6630 W06 ABBOTSFORD 3067 V30 ALDGATE 5154 S18 ANDAS PARK 5353 S19 ARDJORIE STATION 6728 W01 ABBOTSFORD POINT 2046 N21 ALDGATE NORTH 5154 S18 ANDERSON 3995 V31 ARDLETHAN 2665 N29 ABBOTSHAM 7315 T02 ALDGATE PARK 5154 S18 ANDO 2631 N24 ARDMONA 3629 V09 ABERCROMBIE 2795 N19 ALDINGA 5173 S18 ANDOVER 7120 T05 ARDNO 3312 V20 ABERCROMBIE CAVES 2795 N19 ALDINGA BEACH 5173 S18 ANDREWS 5454 S09 ARDONACHIE 3286 V24 ABERDEEN 5417 S15 ALECTOWN 2870 N15 ANEMBO 2621 N24 ARDROSS 6153 W15 ABERDEEN 7310 T02 ALEXANDER PARK 5039 S18 ANGAS PLAINS 5255 S20 ARDROSSAN 5571 S17 ABERFELDY 3825 V33 ALEXANDRA 3714 V14 ANGAS VALLEY 5238 S25 AREEGRA 3480 V02 ABERFOYLE 2350 N03 ALEXANDRA BRIDGE 6288 W18 ANGASTON 5353 S19 ARGALONG 2720 N27 ABERFOYLE PARK 5159 S18 ALEXANDRA HILLS 4161 Q30 ANGEPENA 5732 S05 ARGENTON 2284 N20 ABINGA 5710 18 ALFORD 5554 S16 ANGIP 3393 V02 ARGENTS HILL 2449 N01 ABROLHOS ISLANDS 6532 W06 ALFORDS POINT 2234 N21 ANGLE PARK 5010 S18 ARGYLE 2852 N17 ABYDOS 6721 W02 ALFRED COVE 6154 W15 ANGLE VALE 5117 S18 ARGYLE 3523 V15 ACACIA CREEK 2476 N02 ALFRED TOWN 2650 N29 ANGLEDALE 2550 N43 ARGYLE 6239 W17 ACACIA PLATEAU 2476 N02 ALFREDTON 3350 V26 ANGLEDOOL 2832 N12 ARGYLE DOWNS STATION6743 W01 ACACIA RIDGE 4110 Q30 ALGEBUCKINA -

Leigh Creek Energy Exploration Drilling Operations, PEL 650
Leigh Creek Energy Environmental Impact Report Exploration Drilling Operations Petroleum Exploration Licence 650 PGER 00318 Exploration Drilling Operations EIR 2019 Prepared by: Leigh Creek Energy Limited ACN: 107 531 822 Level 11 19 Grenfell Street Adelaide SA 5000 Phone: 08 8132 9100 Fax: 08 8231 7574 Document Leigh Creek Energy Environmental Impact Report Exploration Drilling Date 29 October 2019 Prepared by Alex Mutiso and John Centofanti, Leigh Creek Energy Limited Revision History Revision Date Purpose Prepared Reviewed Authorised 0 29/10/18 Draft prepared JC/ED (LCK) AM 1 23/11/18 Draft released for SM/BW (JBS&G) AM AM consultation 2 24/10/19 Updated with DN/AM/SA JC AM additional feedback, air quality and groundwater water results 3 29/10/19 Draft released for AM/DN/SA AM AM consultation 4 08/11/19 Issued for submission to JC AM AM DEM 5 28/11/19 Updated with AM JC AM additional feedback 6 07/02/20 Updated following AM/JC AM AM government department reviews 7 13/02/20 Updated following DEM AM AM AM review Leigh Creek Energy Limited ACN: 107 531 82 Page 2 of 156 Exploration Drilling Operations EIR 2019 Leigh Creek Energy acknowledge the Adnyamathanha people, the traditional owners of the land on which our operations occur and pay our respects to their Elders past and present. Leigh Creek Energy Limited ACN: 107 531 82 Page 3 of 156 Exploration Drilling Operations EIR 2019 Contents Summary .................................................................................................................................................