The Decapod Crustaceans of Iowa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Estimating the Threat Posed by the Crayfish Plague Agent
Estimating the threat posed by the crayfish plague agent Aphanomyces astaci to crayfish species of Europe and North America — Introduction pathways, distribution and genetic diversity by Jörn Panteleit from Aachen, Germany Accepted Dissertation thesis for the partial fulfillment of the requirements for a Doctor of Natural Sciences Fachbereich 7: Natur- und Umweltwissenschaften Universität Koblenz-Landau Thesis examiners: Prof. Dr. Ralf Schulz, University of Koblenz-Landau, Germany Dr. Japo Jussila, University of Eastern Finland, Kuopio, Suomi-Finland Date of oral examination: January 17th, 2019 TABLE OF CONTENTS 1. LIST OF PUBLICATIONS ........................................................................................................................ 3 2. ABSTRACT ............................................................................................................................................ 4 2.1 Zusammenfassung ......................................................................................................................... 5 3. ABBREVIATIONS .................................................................................................................................. 6 4. INTRODUCTION ................................................................................................................................... 7 4.1 Invasive species ............................................................................................................................. 7 4.2 Freshwater crayfish in Europe ...................................................................................................... -

Green Ribbon Project
GREEN RIBBON PROJECT (This page is intentionally left blank) GLACIAL LAKES & PRAIRIE ESCAPES OF NORTHWEST IOWA SUPPLEMENTAL PLAN – 2018 Planning & Technical Assistance Provided By: Northwest Iowa Planning & Development Commission 0 | Executive Summary REGIONAL MASTER PLAN GLACIERS & PRAIRIES Many of the celebrated GLACIAL LAKES natural resources in our region were the creation of & glaciers nearly 12,000 years ago. As a result, when the last PRAIRIE ESCAPES glaciers retreated, lakes, marshes, rich soils and OF NORTHWEST IOWA diverse plant and animal life remained. Though the prairies, OUR REGION: WHO WE ARE wetlands, and water formations have been altered SIX NEIGHBORING, YET DIVERSE COUNTIES CAME TOGETHER TO over time, what beauty FORM THE GLACIAL LAKES & PRAIRIE ESCAPES REGION OF remains should be enhanced NORTHWEST IOWA and preserved for generations This includes: of residents and visitors of the BUENA VISTA COUNTY region CLAY COUNTY DICKINSON COUNTY EMMET COUNTY O’BRIEN COUNTY & PALO ALTO COUNTY “The nation behaves well if it treats its natural resources as assets which it must turn over to the next generation increased, and not impaired, in value.” - Theodore Roosevelt Speech to Colorado Livestock Association in Denver on August 29, 1910. i PARTNERS IN PLANNING The Master Plan for the Glacial Lakes & Prairie Escapes The Glacial Lakes Region of Northwest Iowa could not have come to fruition & Prairie Escapes Region without the involvement of community leaders, and public would like thank the efforts of input who lent their time and talents to the completion of this our committee members and project. their associated cities, The plan received input from all 6 counties throughout the counties and organizations. -

Role of Habitat in the Distribution and Abundance of Marsh Birds
s 542 .18 S74 no.;43 1965 Role of Habitat in the Distribution and Abundance of Marsh Birds by Milton W. Weller and Cecil S. Spatcher Department of Zoology and Entomology Special Report No. 43 Agricultural and Home Economics Experiment Station Iowa State University of Science and Technology Ames, Iowa- April 1965 IOWA STATE TRA YEUNG LIBRARY DES MOlNESt 'IOWA CONTENTS Summary ---------------------- -- --------------------------------------- --- ------------------------------ --- ----------- 4 Introduction ------------------- ---- ------ --- -------- ----- ------------------------------ ---------------------- --- ---- 5 Study areas --------- -- --- --- --- -------------------------------- ---------------------- ----------------------- --------- 5 Methods ----------- --- ----------- --------- ------------------------------------------------------- --- -------------------- 6 Vegetation ---------------------------- ------------ --- -------------------------- --- ------------------ -- -------- 6 Bird populations ---------------------------------------------------------------- -------------------------- 6 Results ______ _ __ ____ __ _ __ ___ __ __ __ ______ __ ___ ___ __ _ _ _ ____ __ __ ___ __ ______ __ __ _____ ______ ____ ___ __ _ _ ____ ___ _____ __ __ ___ ___ _ 6 Species composition and chronology of nesting -------------------------------------- 6 Habitat changes at Little Wall and Goose lakes ------ -------------------------------- 8 Bird populations in relation to habitat ----------- ---- ----------- -------------------------- 11 Distribution -
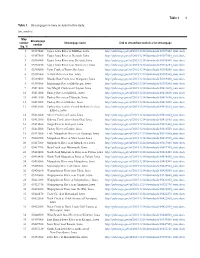
Statistical Summaries of Selected Iowa Streamflow Data--Table 1
Table 1 1 Table 1. Streamgages in Iowa included in this study. [no., number] Map Streamgage number Streamgage name Link to streamflow statistics for streamgage number (fig. 1) 1 05387440 Upper Iowa River at Bluffton, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05387440_stats.docx 2 05387500 Upper Iowa River at Decorah, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05387500_stats.docx 3 05388000 Upper Iowa River near Decorah, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05388000_stats.docx 4 05388250 Upper Iowa River near Dorchester, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05388250_stats.docx 5 05388500 Paint Creek at Waterville, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05388500_stats.docx 6 05389000 Yellow River near Ion, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05389000_stats.docx 7 05389400 Bloody Run Creek near Marquette, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05389400_stats.docx 8 05389500 Mississippi River at McGregor, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05389500_stats.docx 9 05411400 Sny Magill Creek near Clayton, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05411400_stats.docx 10 05411600 Turkey River at Spillville, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05411600_stats.docx 11 05411850 Turkey River near Eldorado, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05411850_stats.docx 12 05412000 Turkey River at Elkader, Iowa http://pubs.usgs.gov/of/2015/1214/downloads/05412000_stats.docx 13 05412020 Turkey River above French Hollow Creek at http://pubs.usgs.gov/of/2015/1214/downloads/05412020_stats.docx -

Little Sioux River Watershed Biotic Stressor Identification Report
Little Sioux River Watershed Biotic Stressor Identification Report April 2015 Authors Editing and Graphic Design Paul Marston Sherry Mottonen Jennifer Holstad Contributors/acknowledgements Michael Koschak Kim Laing The MPCA is reducing printing and mailing costs by Chandra Carter using the Internet to distribute reports and Chuck Regan information to wider audience. Visit our website Mark Hanson for more information. Katherine Pekarek-Scott MPCA reports are printed on 100% post-consumer Colton Cummings recycled content paper manufactured without Tim Larson chlorine or chlorine derivatives. Chessa Frahm Brooke Hacker Jon Lore Cover photo: Clockwise from Top Left: Little Sioux River at site 11MS010; County Ditch 11 at site 11MS078; Cattle around Unnamed Creek at site 11MS067 Project dollars provided by the Clean Water Fund (From the Clean Water, Land and Legacy Amendment) Minnesota Pollution Control Agency 520 Lafayette Road North | Saint Paul, MN 55155-4194 | www.pca.state.mn.us | 651-296-6300 Toll free 800-657-3864 | TTY 651-282-5332 This report is available in alternative formats upon request, and online at www.pca.state.mn.us Document number: wq-ws5-10230003a Contents Executive summary ............................................................................................................... 1 Introduction .......................................................................................................................... 2 Monitoring and assessment ...........................................................................................................2 -

Decapoda: Cambaridae) of Arkansas Henry W
Journal of the Arkansas Academy of Science Volume 71 Article 9 2017 An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas Henry W. Robison Retired, [email protected] Keith A. Crandall George Washington University, [email protected] Chris T. McAllister Eastern Oklahoma State College, [email protected] Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Robison, Henry W.; Crandall, Keith A.; and McAllister, Chris T. (2017) "An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas," Journal of the Arkansas Academy of Science: Vol. 71 , Article 9. Available at: http://scholarworks.uark.edu/jaas/vol71/iss1/9 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas Cover Page Footnote Our deepest thanks go to HWR’s numerous former SAU students who traveled with him in search of crayfishes on many fieldtrips throughout Arkansas from 1971 to 2008. Personnel especially integral to this study were C. -

States of the Lakes
NORTH AMERICAN LAKE NONPROFIT ORG. MANAGEMENT SOCIETY US POSTAGE 1315 E. Tenth Street PAID Bloomington, IN 47405-1701 Bloomington, IN Permit No. 171 States of the Lakes the of States L L INE Volume 36, No. 4 • Winter 2016 Winter • 4 No. 36, Volume AKE A publication of the North American Lake Management Society Society Management Lake American North the of publication A AKE INE Contents L L Published quarterly by the North American Lake Management Society (NALMS) as a medium for exchange and communication among all those Volume 36, No. 4 / Winter 2016 interested in lake management. Points of view expressed and products advertised herein do not necessarily reflect the views or policies of 2 From the Editor NALMS or its Affiliates. Mention of trade names and commercial products shall not constitute 4 From the President an endorsement of their use. All rights reserved. Standard postage is paid at Bloomington, IN and 6 2016 NALMS Symposium Summary additional mailing offices. 9 2016 NALMS Awards NALMS Officers President 14 2016 NALMS By the Numbers Frank Wilhelm Immediate Past-President 15 2016 NALMS Photo Contest Results Julie Chambers 16 2016 NALMS Election Results President-Elect Frank Browne Secretary Amy Smagula States of the States Treasurer Michael Perry 18 Tropical Storm Irene & the Passage of Vermont’s Shoreland Protection Act NALMS Regional Directors 21 Region 1 Perry Thomas Wisconsin Lake Management, Programs, Policy, and Region 2 Kiyoko Yokota Litigation Region 3 Lisa Borre Region 4 Diane Lauritsen 24 Lake Restoration in Iowa: Building Partnerships for Success Region 5 Eugene Braig Region 6 Brad Hufhines 30 Top-Down Water Quality Approach in Texas Region 7 George Antoniou Region 8 Mike Eytel Region 9 Ellen Preece 31 An Overview of Lake Monitoring and Management in Region 10 Shannon Brattebo Colorado Region 11 Anna DeSellas Region 12 John-Mark Davies 35 At-Large Sara Peel Lake Management In Ontario Student At-Large Sarah Burnet LakeLine Staff Editor: William W. -

Ground Water in Alluvial Channel Deposits Nobles County, Minnesota
Bulletin No. 14 DIVISION OF WATERS MINNESOTA DEPARTMENT OF CONSERVATION GROUND WATER IN ALLUVIAL CHANNEL DEPOSITS NOBLES COUNTY, MINNESOTA By Ralph F. Norvitch U. S. Geological Survey Prepared cooperatively by the Geological Survey, U. S. Department of the Interior and the Division of Waters, Minnesota Department of Conservation St. Paul, Minn. September 1960 1 CONTENTS Page Abstract……………………………………………………………………………………3 Introduction………………………………………………………………………………..4 Geology……………………………………………………………………………………4 History of the valleys……………………………………………………………...5 Thickness of the alluvium…………………………………………………………7 Texture of the alluvium…………………………………………………………..10 Ground water conditions…………………………………………………………………11 Significant factors for locating wells…………………………………………………….13 Quality of water………………………………………………………………………….14 Conclusions………………………………………………………………………………14 References………………………………………………………………………………..15 ILLUSTRATIONS Figure 1. Map of Nobles County, Minn., showing alluvial deposits, morainal fronts, auger holes, selected municipal wells, and the Missouri-Mississippi River divide …………………………………………16 2. Generalized cross section of Little Rock River valley, Nobles County………..9 TABLES Table 1. Data from auger holes bored in the alluvial deposits in Nobles County, Minn. ………………………………………….8 2. Summary of data from auger holes bored in the alluvial deposits in Nobles County………………………………………………………………………….10 2 GROUND WATER IN ALLUVIAL CHANNEL DEPOSITS NOBLES COUNTY, MINNESOTA By Ralph F. Norvitch ABSTRACT The alluvial channel deposits described in this report are in Nobles County, Minn., about 150 miles southwest of Minneapolis and St. Paul. Although four municipalities and many farms obtain part or all of their water needs from the alluvium, it has not yet been fully developed for ground water. The extent of the alluvial channel deposits was mapped on high-altitude aerial photographs, and a power auger was used to bore 43 test holes to determine the thickness of alluvium and the water level at each of the test sites. -

The Pacific Northwest As an Emerging Beachhead of Crayfish Invasions
Oregon Invasive Species Council (Oct 2019) The Pacific Northwest as an emerging beachhead of crayfish invasions Julian D. Olden University of Washington Oregon Invasive Species Council @oldenfish October 15-16, 2019 [email protected] Signal crayfish Pacifastacus leniusculus Julian Olden (UW, [email protected]) 1 Oregon Invasive Species Council (Oct 2019) Chronology of crayfish invasions in the western U.S. P. clarkii F. neglectus F. sanbornii Oregon, Washington F. rusticus Oregon Washington Mueller (2001), Pearl et al. (2005), Oregon Bouchard (1977) Larson et al. (2010) Larson and Olden (2008) Olden et al. (2009) 1960 1970 1980 1990 2000 2010 P. clarkii F. virilis F. virilis P. acutus Idaho Montana Idaho, Washington Washington Clark and Wroten (1978) Sheldon (1988) Clark and Lester (2005), Larson and Olden (2011) Larson and Olden (2008), Larson et al. (2010) Non-native Crayfish in the PNW Red swamp crawfish Northern crayfish Procambarus clarkii Faxonius virilis Ringed crayfish Rusty crayfish Faxonius rusticus Faxonius rusticus Julian Olden (UW, [email protected]) 2 Oregon Invasive Species Council (Oct 2019) Pathways of Crayfish Introductions • Release as live bait associated with angling • Release after educational use in classroom • Release associated with pet aquariums • Intentional introduction as forage for sportfish • Intentional introduction to golf course ponds • Intentional introduction to create harvest opportunities • Secondary spread from existing populations Impacts of Invasive Crayfish Meta-analysis of the published literature demonstrates -

Ecological Effects of Predator Information Mediated by Prey Behavior
ECOLOGICAL EFFECTS OF PREDATOR INFORMATION MEDIATED BY PREY BEHAVIOR Tyler C. Wood A Dissertation Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY May 2020 Committee: Paul Moore, Advisor Robert Green Graduate Faculty Representative Shannon Pelini Andrew Turner Daniel Wiegmann ii ABSTRACT Paul Moore, Advisor The interactions between predators and their prey are complex and drive much of what we know about the dynamics of ecological communities. When prey animals are exposed to threatening stimuli from a predator, they respond by altering their morphology, physiology, or behavior to defend themselves or avoid encountering the predator. The non-consumptive effects of predators (NCEs) are costly for prey in terms of energy use and lost opportunities to access resources. Often, the antipredator behaviors of prey impact their foraging behavior which can influence other species in the community; a process known as a behaviorally mediated trophic cascade (BMTC). In this dissertation, predator odor cues were manipulated to explore how prey use predator information to assess threats in their environment and make decisions about resource use. The three studies were based on a tri-trophic interaction involving predatory fish, crayfish as prey, and aquatic plants as the prey’s food. Predator odors were manipulated while the foraging behavior, shelter use, and activity of prey were monitored. The abundances of aquatic plants were also measured to quantify the influence of altered crayfish foraging behavior on plant communities. The first experiment tested the influence of predator odor presence or absence on crayfish behavior. -

First Report of Golden Crayfish Faxonius Luteus (Creaser, 1933) in South Dakota
BioInvasions Records (2021) Volume 10, Issue 1: 149–157 CORRECTED PROOF Rapid Communication First report of golden crayfish Faxonius luteus (Creaser, 1933) in South Dakota Gene Galinat1,*, Mael Glon2 and Brian Dickerson3 1South Dakota Department of Game, Fish and Parks, Rapid City, South Dakota, USA 2The Ohio State University Museum of Biological Diversity, Columbus, Ohio, USA 3U.S. Department of Agriculture Forest Service, Rocky Mountain Research Station, Rapid City, SD, 57702, USA Author e-mails: [email protected] (GG), [email protected] (MG), [email protected] (BD) *Corresponding author Citation: Galinat G, Glon M, Dickerson B (2021) First report of golden crayfish Abstract Faxonius luteus (Creaser, 1933) in South Dakota. BioInvasions Records 10(1): 149– The golden crayfish, Faxonius luteus, was identified for the first time in the Black 157, https://doi.org/10.3391/bir.2021.10.1.16 Hills of South Dakota. We collected specimens from three reservoirs and one stream in two adjacent watersheds. The species appears to be established with varying Received: 7 February 2020 sizes and Form I and Form II males being observed. Records show the home range Accepted: 20 August 2020 of F. luteus to be over 600 km east of the Black Hills. The lack of historic information Published: 1 December 2020 on aquatic fauna in the area complicates determining what effects F. luteus may Handling editor: David Hudson have on native and other non-native fauna in the area. Thematic editor: Karolina Bącela- Spychalska Key words: bait, baitfish, Black Hills, non-native Copyright: © Galinat et al. -

Recent Research Into Invasive Crayfish of the Carolinas
Recent research into invasive crayfishes of the Carolinas Michael Kendrick South Carolina DNR [email protected] 38 >385 0 64 9 143 38 >385 0 64 9 143 High diversity centered in southeast US 40 Species of Crayfish in South Carolina Procambarus acutus Creaserinus fodiens Data: updated from Eversole & Foltz 2015 Spread and impacts of non-native crayfish 1. Spread: 1. Current distributions of key invasive crayfish 2. Impacts: 1. Potential for hybridization 2. Disease vectors Native crayfish transplants in the US 1. Rusty crayfish 14. Kentucky river crayfish 26. Seminole crayfish 2. Virile crayfish 15. Golden crayfish 27. Southern white river 3. Red swamp crayfish 16. Ringed crayfish crayfish 4. Cajun dwarf crayfish 17. Gap ringed crayfish 5. Cumberland crayfish 18. Allegheny crayfish 6. Longnose crayfish 19. Creole painted crayfish 7. Big water crayfish 20. Northern clearwater 8. Ditch fencing crayfish crayfish 9. Western plains crayfish 21. Sanborn crayfish 10. Spiny stream crayfish 22. Conchas crayfish 11. Belted crayfish 23. White River crayfish 12. Woodland crayfish 24. Everglades crayfish 13. Calico crayfish 25. Straightedge crayfish Source: USGS NAS Database Native crayfish transplant threats to SC 1. Rusty crayfish 2. Virile crayfish 3. Red swamp crayfish Rusty crayfish Virile crayfish Red swamp crayfish Source: USGS NAS Database Virile crayfish (Faxonius virilis) in the U.S. • Prefer streams with moderate flow • Bait introduction and intentional stocking for forage Rusty crayfish (Faxonius rusticus) in the U.S. (near SC/NC border) • Inhabits lakes, ponds, and streams. • Bait introduction and school pets • Out-compete and displace native crayfish and reduce resources Red swamp crayfish (P.