Baseline Functional Connectivity in Resting State Networks Associated with Depression and Remission
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Role for the Left Angular Gyrus in Episodic Simulation and Memory
8142 • The Journal of Neuroscience, August 23, 2017 • 37(34):8142–8149 Behavioral/Cognitive A Role for the Left Angular Gyrus in Episodic Simulation and Memory X Preston P. Thakral, XKevin P. Madore, and XDaniel L. Schacter Department of Psychology, Harvard University, Boston, Massachusetts 02138 Functional magnetic resonance imaging (fMRI) studies indicate that episodic simulation (i.e., imagining specific future experiences) and episodic memory (i.e., remembering specific past experiences) are associated with enhanced activity in a common set of neural regions referred to as the core network. This network comprises the hippocampus, medial prefrontal cortex, and left angular gyrus, among other regions. Because fMRI data are correlational, it is unknown whether activity increases in core network regions are critical for episodic simulation and episodic memory. In the current study, we used MRI-guided transcranial magnetic stimulation (TMS) to assess whether temporary disruption of the left angular gyrus would impair both episodic simulation and memory (16 participants, 10 females). Relative to TMS to a control site (vertex), disruption of the left angular gyrus significantly reduced the number of internal (i.e., episodic) details produced during the simulation and memory tasks, with a concomitant increase in external detail production (i.e., semantic, repetitive, or off-topic information), reflected by a significant detail by TMS site interaction. Difficulty in the simulation and memory tasks also increased after TMS to the left angular gyrus relative to the vertex. In contrast, performance in a nonepisodic control task did not differ statistically as a function of TMS site (i.e., number of free associates produced or difficulty in performing the free associate task). -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Functional Connectivity of the Angular Gyrus in Normal Reading and Dyslexia (Positron-Emission Tomography͞human͞brain͞regional͞cerebral)
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 8939–8944, July 1998 Neurobiology Functional connectivity of the angular gyrus in normal reading and dyslexia (positron-emission tomographyyhumanybrainyregionalycerebral) B. HORWITZ*†,J.M.RUMSEY‡, AND B. C. DONOHUE‡ *Laboratory of Neurosciences, National Institute on Aging, and ‡Child Psychiatry Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892 Communicated by Robert H. Wurtz, National Eye Institute, Bethesda, MD, May 14, 1998 (received for review January 19, 1998) ABSTRACT The classic neurologic model for reading, not functionally connected during a specific task. On the other based on studies of patients with acquired alexia, hypothesizes hand, if rCBF in two regions is correlated, these regions need functional linkages between the angular gyrus in the left not be anatomically linked; their activities may be correlated, hemisphere and visual association areas in the occipital and for example, because both receive inputs from a third area (for temporal lobes. The angular gyrus also is thought to have more discussion about these connectivity concepts, see refs. 8, functional links with posterior language areas (e.g., Wer- 10, and 11). nicke’s area), because it is presumed to be involved in mapping Based on lesion studies in many patients with alexia, it has visually presented inputs onto linguistic representations. Us- been proposed that the posterior portion of the neural network ing positron emission tomography , we demonstrate in normal mediating reading in the left cerebral hemisphere involves men that regional cerebral blood flow in the left angular gyrus functional links between the angular gyrus and extrastriate shows strong within-task, across-subjects correlations (i.e., areas in occipital and temporal cortex associated with the functional connectivity) with regional cerebral blood flow in visual processing of letter and word-like stimuli (12–14). -

Seed MNI Coordinates Lobe
MNI Coordinates Seed Lobe (Hemisphere) Region BAa X Y Z FP1 -18 62 0 Frontal Lobe (L) Medial Frontal Gyrus 10 FPz 4 62 0 Frontal Lobe (R) Medial Frontal Gyrus 10 FP2 24 60 0 Frontal Lobe (R) Superior Frontal Gyrus 10 AF7 -38 50 0 Frontal Lobe (L) Middle Frontal Gyrus 10 AF3 -30 50 24 Frontal Lobe (L) Superior Frontal Gyrus 9 AFz 4 58 30 Frontal Lobe (R) Medial Frontal Gyrus 9 AF4 36 48 20 Frontal Lobe (R) Middle Frontal Gyrus 10 AF8 42 46 -4 Frontal Lobe (R) Inferior Frontal Gyrus 10 F7 -48 26 -4 Frontal Lobe (L) Inferior Frontal Gyrus 47 F5 -48 28 18 Frontal Lobe (L) Inferior Frontal Gyrus 45 F3 -38 28 38 Frontal Lobe (L) Precentral Gyrus 9 F1 -20 30 50 Frontal Lobe (L) Superior Frontal Gyrus 8 Fz 2 32 54 Frontal Lobe (L) Superior Frontal Gyrus 8 F2 26 32 48 Frontal Lobe (R) Superior Frontal Gyrus 8 F4 42 30 34 Frontal Lobe (R) Precentral Gyrus 9 F6 50 28 14 Frontal Lobe (R) Middle Frontal Gyrus 46 F8 48 24 -8 Frontal Lobe (R) Inferior Frontal Gyrus 47 FT9 -50 -6 -36 Temporal Lobe (L) Inferior Temporal Gyrus 20 FT7 -54 2 -8 Temporal Lobe (L) Superior Temporal Gyrus 22 FC5 -56 4 22 Frontal Lobe (L) Precentral Gyrus 6 FC3 -44 6 48 Frontal Lobe (L) Middle Frontal Gyrus 6 FC1 -22 6 64 Frontal Lobe (L) Middle Frontal Gyrus 6 FCz 4 6 66 Frontal Lobe (R) Medial Frontal Gyrus 6 FC2 28 8 60 Frontal Lobe (R) Sub-Gyral 6 FC4 48 8 42 Frontal Lobe (R) Middle Frontal Gyrus 6 FC6 58 6 16 Frontal Lobe (R) Inferior Frontal Gyrus 44 FT8 54 2 -12 Temporal Lobe (R) Superior Temporal Gyrus 38 FT10 50 -6 -38 Temporal Lobe (R) Inferior Temporal Gyrus 20 T7/T3 -

Angular Gyrus Involvement at Encoding and Retrieval Is Associated with Durable but Less Specific Memories
9474 • The Journal of Neuroscience, September 27, 2017 • 37(39):9474–9485 Behavioral/Cognitive Angular Gyrus Involvement at Encoding and Retrieval Is Associated with Durable But Less Specific Memories Marieke van der Linden,1 XRuud M.W.J. Berkers,1 XRichard G.M. Morris,2 and Guille´n Ferna´ndez1 1Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen Medical Centre, 6500 HB Nijmegen, The Netherlands, and 2Centre for Cognitive and Neural Systems, The University of Edinburgh, EH8 9JZ Edinburgh, United Kingdom Afterconsolidation,informationbelongingtoamentalschemaisbetterremembered,butsuchmemorycanbelessspecificwhenitcomes to details. A neuronal mechanism consistent with this behavioral pattern could result from a dynamic interaction that entails mediation byaspecificcorticalnetworkwithassociatedhippocampaldisengagement.Wenowreportthat,inmaleandfemaleadulthumansubjects, encoding and later consolidation of a series of objects embedded in a semantic schema was associated with a buildup of activity in the angular gyrus (AG) that predicted memory 24 h later. In parallel, the posterior hippocampus became less involved as schema objects were encoded successively. Hippocampal disengagement was related to an increase in falsely remembering objects that were not presented at encoding. During both encoding and retrieval, the AG and lateral occipital complex (LOC) became functionally connected and this interaction was beneficial for successful retrieval. Therefore, a network including the AG and LOC enhances the overnight retention of schema-related memories and their simultaneous detachment from the hippocampus reduces the specificity of the memory. Key words: angular gyrus; fMRI; hippocampus; memory; schema Significance Statement This study provides the first empirical evidence on how the hippocampus and the neocortex interact dynamically when acquiring and then effectively retaining durable knowledge that is associated to preexisting knowledge, but they do so at the cost of memory specificity. -

Functional Connectivity of the Precuneus in Unmedicated Patients with Depression
Biological Psychiatry: CNNI Archival Report Functional Connectivity of the Precuneus in Unmedicated Patients With Depression Wei Cheng, Edmund T. Rolls, Jiang Qiu, Deyu Yang, Hongtao Ruan, Dongtao Wei, Libo Zhao, Jie Meng, Peng Xie, and Jianfeng Feng ABSTRACT BACKGROUND: The precuneus has connectivity with brain systems implicated in depression. METHODS: We performed the first fully voxel-level resting-state functional connectivity (FC) neuroimaging analysis of depression of the precuneus, with 282 patients with major depressive disorder and 254 control subjects. RESULTS: In 125 unmedicated patients, voxels in the precuneus had significantly increased FC with the lateral orbitofrontal cortex, a region implicated in nonreward that is thereby implicated in depression. FC was also increased in depression between the precuneus and the dorsolateral prefrontal cortex, temporal cortex, and angular and supramarginal areas. In patients receiving medication, the FC between the lateral orbitofrontal cortex and precuneus was decreased back toward that in the control subjects. In the 254 control subjects, parcellation revealed superior anterior, superior posterior, and inferior subdivisions, with the inferior subdivision having high connectivity with the posterior cingulate cortex, parahippocampal gyrus, angular gyrus, and prefrontal cortex. It was the ventral subdivision of the precuneus that had increased connectivity in depression with the lateral orbitofrontal cortex and adjoining inferior frontal gyrus. CONCLUSIONS: The findings support the theory that the system in the lateral orbitofrontal cortex implicated in the response to nonreceipt of expected rewards has increased effects on areas in which the self is represented, such as the precuneus. This may result in low self-esteem in depression. The increased connectivity of the precuneus with the prefrontal cortex short-term memory system may contribute to the rumination about low self-esteem in depression. -

Stimulation of the Angular Gyrus Improves the Level of Consciousness
brain sciences Article Stimulation of the Angular Gyrus Improves the Level of Consciousness Liudmila Legostaeva *, Alexandra Poydasheva , Elizaveta Iazeva , Dmitry Sinitsyn , Dmitry Sergeev , Ilya Bakulin, Dmitry Lagoda, Elena Kremneva , Sofya Morozova , Yulia Ryabinkina, Natalia Suponeva and Michael Piradov Research Center of Neurology, Volokolamskoe shosse, 80, Moscow 125367, Russia; [email protected] (A.P.); [email protected] (E.I.); [email protected] (D.S.); [email protected] (D.S.); [email protected] (I.B.); [email protected] (D.L.); [email protected] (E.K.); [email protected] (S.M.); [email protected] (Y.R.); [email protected] (N.S.); [email protected] (M.P.) * Correspondence: [email protected]; Tel.: +79672974443 Received: 19 March 2019; Accepted: 5 May 2019; Published: 6 May 2019 Abstract: Background: Navigated repetitive transcranial magnetic stimulation (rTMS) is a promising tool for neuromodulation. In previous studies it has been shown that the activity of the default mode network (DMN) areas, particularly of its key region—the angular gyrus—is positively correlated with the level of consciousness. Our study aimed to explore the effect of rTMS of the angular gyrus as a new approach for disorders of consciousness (DOC) treatment; Methods: A 10-session 2-week high-frequency rTMS protocol was delivered over the left angular gyrus in 38 DOC patients with repeated neurobehavioral assessments obtained at baseline and in 2 days after the stimulation course was complete; Results: 20 Hz-rTMS over left angular gyrus improved the coma recovery scale revised (CRS-R) total score in minimally conscious state (MCS) patients. We observed no effects in vegetative state (VS) patients; and Conclusions: The left angular gyrus is likely to be effective target for rTMS in patients with present signs of consciousness. -

Supplementary File 1
Supplementary Material Table S1. Means and standard deviations for 45 emotional pictures used in the fMRI task. Category Picture code Valence Arousal Dominance M (SD) M (SD) M (SD) Death Graveyard (x8); Cadaver (x2); 2.27(0.37) 5.56 (0.60) 5.52 (0.44) Terminal disease (x3); Accident (x2) Negative 2682; 2692; 2722; 2751; 2752; 3180; 2.47(0.48) 5.61 (0.91) 3.41 (0.82) Valence 6010; 6821; 9041; 9080; 9120; 9290; 9415; 9530; 9560; Positive 1463; 2010; 2299; 2346; 2360; 4614; 7.68(0.42) 5.04 (0.91) 5.78 (0.64) Valence 4622; 5260; 7220; 7502; 8190; 8380; 8461; 8496; 8600 Note: M = Mean, SD = Standard Deviation. Neuroimaging within group results Death pictures > fixation cross In the contrast of death pictures with the fixation cross, both groups showed bilateral activation in the occipital cortex, intraparietal cortex, and fusiform gyri. The CG group also showed activation in the amygdala, hippocampus giri, thalamus, anterior insula, orbitofrontal cortices, middle frontal giri, supplementary motor area, anterior cingulate cortex, and right putamen. The NB group (but not CG group) showed significant deactivation of areas of the default mode network (i.e., medial prefrontal cortex and angular gyrus), the anterior cingulate cortex, and the superior temporal cortices extending to the posterior insula (see Table SM2). Table S2. Brain regions showing significant activation or deactivation associated with the processing of death-related pictures. CG NB Brain region X Y Z kE t value X Y Z kE t value Death pictures > Fixation Cross Activations Occipital -

A Meta-Analysis
www.nature.com/scientificreports OPEN Information content best characterises the hemispheric selectivity of the inferior parietal lobe: a meta‑analysis Oliver Gray*, Lewis Fry & Daniela Montaldi Our understanding of the inferior parietal lobe (IPL) remains challenged by inconsistencies between neuroimaging and neuropsychological perspectives. To date, others assume that hemispheric specialisation of the IPL is linked with the type of processing; attention processing in the right hemisphere; memory retrieval and semantic judgement in the left hemisphere. Here, we provide compelling evidence associating the type of information being processed with the recruitment of each hemisphere’s IPL. In a meta-analysis, we classify 121 previous fMRI reports of IPL activity arising from episodic memory retrieval, according to the type of information that characterises each fMRI contrast. We demonstrate that the left IPL is more consistently associated with retrieval of the semantic (95% of eligible contrasts) than perceptual aspects of memory (83%). In contrast, the right IPL is more consistently associated with the retrieval of perceptual (97%), than semantic aspects of memory (43%). This work revises assumptions of how the IPL contributes to healthy cognition and has major implications for IPL-related neuropsychological defcits. Our understanding of the complex brain systems that support the encoding, storage, and retrieval of episodic memory, the what, where, and when of life’s events, has vastly improved with the advent of neuroimaging. However, for the last 15 years, the functional role played by the inferior parietal lobule (IPL), which includes the angular gyrus and supramarginal gyrus, in the retrieval of episodic memory has been the source of contentious debate1–6. -
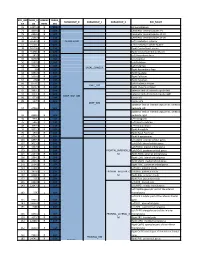
Core-Example1.Pdf
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital -

Title: Angular Default Mode Network Connectivity Across Working Memory Load
Deniz Vatansever Angular Default Network in Working Memory Title: Angular Default Mode Network Connectivity Across Working Memory Load Short title: Angular Default Network in Working Memory D Vatansever1, AE Manktelow1, BJ Sahakian2, DK Menon1, EA Stamatakis1 1 Division of Anaesthesia and Department of Clinical Neurosciences, School of Clinical Medicine, University of Cambridge, Cambridge, UK 2 Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, UK 3 Department of Psychiatry, School of Clinical Medicine, University of Cambridge, Cambridge, UK Keywords: default mode network, angular gyrus, intrinsic connectivity contrast, functional connectivity, working memory, behavioral performance, n-back Correspondence Details: Deniz Vatansever, MSc, BSc Department of Clinical Neurosciences Division of Anaesthesia University of Cambridge Box 93, Addenbrooke's Hospital Hills Road, Cambridge, UK CB2 0QQ E-mail: [email protected] Tel: +44 (0) 1223 217892 Fax: ++ 44 (0) 1223 217887 Website: http://www.neuroscience.cam.ac.uk/directory/profile.php?ddsv2 Deniz Vatansever Angular Default Network in Working Memory Abstract Initially identified during no-task, Baseline conditions, it has now Been suggested that the default mode network (DMN) engages during a variety of working memory paradigms through its flexiBle interactions with other large-scale Brain networks. Nevertheless, its contriBution to whole-brain connectivity dynamics across increasing working memory load has not Been explicitly assessed. The aim of our study was to determine which DMN huBs relate to working memory task performance during an fMRI-based n-back paradigm with parametric increases in difficulty. Using a voxel-wise metric, termed the intrinsic connectivity contrast (ICC), we found that the Bilateral angular gyri (core DMN hubs) displayed the greatest change in global connectivity across three levels of n-back task load. -

Dissociable Connectivity Within Human Angular Gyrus and Intraparietal Sulcus: Evidence from Functional and Structural Connectivity
Cerebral Cortex Advance Access published February 12, 2010 Cerebral Cortex doi:10.1093/cercor/bhq011 Dissociable Connectivity within Human Angular Gyrus and Intraparietal Sulcus: Evidence from Functional and Structural Connectivity Lucina Q. Uddin1, Kaustubh Supekar1,2, Hitha Amin1, Elena Rykhlevskaia1,3,4, Daniel A. Nguyen1, Michael D. Greicius4 and Vinod Menon1,4,5 1Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA, 2Graduate Program in Biomedical Informatics, Stanford University School of Medicine, Stanford, CA 94305, USA, 3Department of Psychology, Stanford University, Stanford, CA 94305, USA, 4Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA and 5Program in Neuroscience, Stanford University School of Medicine, Stanford, CA 94304, USA Address correspondence to Lucina Q. Uddin, 780 Welch Road, Room 201, Stanford, CA 94304, USA. Email: [email protected] The inferior parietal lobule (IPL) of the human brain is a heterogeneous cortical areas not present in the monkey, others have argued region involved in visuospatial attention, memory, and mathematical that the IPL is similar across both species (Husain and Nachev cognition. Detailed description of connectivity profiles of subdivisions 2007). Thus, it is unclear to what degree monkey anatomical within the IPL is critical for accurate interpretation of functional tracer studies can be extrapolated to understanding these neuroimaging studies involving this region. We separately examined pathways in the human brain. functional and structural connectivity of the angular gyrus (AG) and The human IPL consists of 3 prominent functional and the intraparietal sulcus (IPS) using probabilistic cytoarchitectonic anatomical subdivisions, the angular gyrus (AG), the supra- maps.