Immunogenicity and Efficacy Testing in Chimpanzees of an Oral Hepatitis B Vaccine Based on Live Recombinant Adenovirus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hepatitis B Fast Facts Everything You Need to Know in 2 Minutes Or Less!
Hepatitis B Foundation Cause for a Cure www.hepb.org Hepatitis B Fast Facts Everything you need to know in 2 minutes or less! Hepatitis B is the most common serious liver infection in the world. It is caused by the hepatitis B virus (HBV) that attacks liver cells and can lead to liver failure, cirrhosis (scarring) or cancer of the liver. The virus is transmitted through contact with blood and bodily fluids that contain blood. Most people are able to fight off the hepatitis B infection and clear the virus from their blood. This may take up to six months. While the virus is present in their blood, infected people can pass the virus on to others. Approximately 5-10% of adults, 30-50% of children, and 90% of babies will not get rid of the virus and will develop chronic infection. Chronically infected people can pass the virus on to others and are at increased risk for liver problems later in life. The hepatitis B virus is 100 times more infectious than the AIDS virus. Yet, hepatitis B can be pre- vented with a safe and effective vaccine. For the 400 million people worldwide who are chronically infected with hepatitis B, the vaccine is of no use. However, there are promising new treatments for those who live with chronic hepatitis B. In the World: • This year alone, 10 to 30 million people will become infected with the hepatitis B virus (HBV). • The World Health Organization estimates that 400 million people worldwide are already chronically infected with hepatitis B. -

Prevention & Control of Viral Hepatitis Infection
Prevention & Control of Viral Hepatitis Infection: A Strategy for Global Action © World Health Organization 2011. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. Table of contents Disease burden 02 What is viral hepatitis? 05 Prevention & control: a tailored approach 06 Global Achievements 08 Remaining challenges 10 World Health Assembly: a mandate for comprehensive prevention & control 13 WHO goals and strategy -

Hepatitis B? HEPATITIS B Hepatitis B Is a Contagious Liver Disease That Results from Infection with the Hepatitis B Virus
What is Hepatitis B? HEPATITIS B Hepatitis B is a contagious liver disease that results from infection with the Hepatitis B virus. When first infected, a person can develop Are you at risk? an “acute” infection, which can range in severity from a very mild illness with few or no symptoms to a serious condition requiring hospitalization. Acute Hepatitis B refers to the first 6 months after someone is exposed to the Hepatitis B virus. Some people are able to fight the infection and clear the virus. For others, the infection remains and leads to a “chronic,” or lifelong, illness. Chronic Hepatitis B refers to the illness that occurs when the Hepatitis B virus remains in a person’s body. Over time, the infection can cause serious health problems. How is Hepatitis B spread? Hepatitis B is usually spread when blood, semen, or other body fluids from a person infected with the Hepatitis B virus enter the body of someone who is not infected. This can happen through having sex with an infected partner; sharing needles, syringes, or other injection drug equipment; or from direct contact with the blood or open sores of an infected person. Hepatitis B can also be passed from an infected mother to her baby at birth. Who should be tested for Hepatitis B? Approximately 1.2 million people in the United States and 350 million people worldwide have Hepatitis B. Testing for Hepatitis B is recommended for certain groups of people, including: Most are unaware of their infection. ■ People born in Asia, Africa, and other regions with moderate or high rates Is Hepatitis B common? of Hepatitis B (see map) Yes. -

Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Understanding Human Astrovirus from Pathogenesis to Treatment
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 6-2020 Understanding Human Astrovirus from Pathogenesis to Treatment Virginia Hargest University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Diseases Commons, Medical Sciences Commons, and the Viruses Commons Recommended Citation Hargest, Virginia (0000-0003-3883-1232), "Understanding Human Astrovirus from Pathogenesis to Treatment" (2020). Theses and Dissertations (ETD). Paper 523. http://dx.doi.org/10.21007/ etd.cghs.2020.0507. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Understanding Human Astrovirus from Pathogenesis to Treatment Abstract While human astroviruses (HAstV) were discovered nearly 45 years ago, these small positive-sense RNA viruses remain critically understudied. These studies provide fundamental new research on astrovirus pathogenesis and disruption of the gut epithelium by induction of epithelial-mesenchymal transition (EMT) following astrovirus infection. Here we characterize HAstV-induced EMT as an upregulation of SNAI1 and VIM with a down regulation of CDH1 and OCLN, loss of cell-cell junctions most notably at 18 hours post-infection (hpi), and loss of cellular polarity by 24 hpi. While active transforming growth factor- (TGF-) increases during HAstV infection, inhibition of TGF- signaling does not hinder EMT induction. However, HAstV-induced EMT does require active viral replication. -

Hepatitis B Factsheet: COVID-19 and Hep B
Hepatitis B factsheet: COVID-19 and hep B For more information about anything in this factsheet, phone the Hepatitis Infoline on 1800 803 990 or go to www.hep.org.au COVID-19 and hep B Frequently Asked Questions (FAQ) There’s so much information swirling around these days as we deal with the changing world around us due to COVID-19. There’s lots of incorrect information and it’s easy to be overwhelmed and confused. On this page, we’ll try to clear some things up. It is important that information is helpful, clear, and checked and confirmed by experts. Please share the following information – it comes from health and medical specialists. COVID-19 is an acronym that stands for COronaVIrus Disease 2019. You might hear it called coronavirus, corona, SARS-CoV-2, or even rona. We’ll call it COVID-19 or coronavirus in this FAQ but all these terms are essentially talking about the same thing and we’ll explain them at the bottom. You’ll also hear new or unfamiliar terms like “social distancing,” “physical distancing,” “self-isolation,” and “quarantine”. These terms and what they actually mean can become confusing so we’ve including a glossary at the bottom of this FAQ. This virus has only been known about since around December 2019 so it’s all very new, information changes quickly, and we’re all learning day-by-day and week-by-week. Compare COVID-19 to hep B which we’ve known about since the 1980s and hep B even earlier in the 1960s! We’ll regularly update this page as new information comes to light. -

Interaction Between Hepatitis B Virus and SARS-Cov-2 Infections
World Journal of W J G Gastroenterology Submit a Manuscript: https://www.f6publishing.com World J Gastroenterol 2021 March 7; 27(9): 782-793 DOI: 10.3748/wjg.v27.i9.782 ISSN 1007-9327 (print) ISSN 2219-2840 (online) MINIREVIEWS Interaction between hepatitis B virus and SARS-CoV-2 infections Tian-Dan Xiang, Xin Zheng ORCID number: Tian-Dan Xiang Tian-Dan Xiang, Xin Zheng, Department of Infectious Diseases, Union Hospital, Tongji Medical 0000-0003-2792-6631; Xin Zheng College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, 0000-0001-6564-7807. China Author contributions: Zheng X Corresponding author: Xin Zheng, MD, PhD, Professor, Department of Infectious Diseases, contributed to the conception and Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, No. design of the work and revised the 1277 Jiefang Avenue, Wuhan 430022, Hubei Province, China. [email protected] manuscript; Xiang TD performed the literature review and drafted the manuscript. Abstract Conflict-of-interest statement: Coronavirus disease 2019 (COVID-19) has become a global pandemic and Authors declare no conflict of garnered international attention. The causative pathogen of COVID-19 is severe interest for this article. acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel, highly contagious coronavirus. Numerous studies have reported that liver injury is quite Open-Access: This article is an common in patients with COVID-19. Hepatitis B has a worldwide distribution as open-access article that was well as in China. At present, hepatitis B virus (HBV) remains a leading cause of selected by an in-house editor and cirrhosis, liver failure, and hepatocellular carcinoma. -

Hepatitis B Virus (HBV)
HEPATITIS B AND COLLEGE STUDENTS Hepatitis B is a liver disease that results from infection with the Hepatitis B virus (HBV). It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis B is usually spread when blood, semen, or another body fluid from a person infected with the Hepatitis B virus enters the body of someone who is not infected. This can happen through sexual contact with an infected person or sharing needles, syringes, or other drug-injection equipment. Hepatitis B can also be passed from an infected mother to her baby at birth. Hepatitis B can be either acute or chronic. Acute HBV infection is a short-term illness that occurs within the first 6 months after someone is exposed to the Hepatitis B virus. Acute infection can — but does not always — lead to chronic infection. Chronic HBV infection is a long-term illness that occurs when the Hepatitis B virus remains in a person’s body. Chronic HBV is a serious disease that can result in long-term health problems, and even death. The best way to prevent HBV infection is by getting vaccinated. How common is hepatitis B in the United States? About 800,000 to 1.4 million persons in the United States have chronic HBV infection. Each year 38,000 more people, mostly young adults, get infected with HBV and almost 2,000 people die from chronic HBV. How is hepatitis B spread? Hepatitis B is spread when blood, semen, or other body fluid infected with the hepatitis B virus enters the body of a person who is not infected. -

Arenaviridae Astroviridae Filoviridae Flaviviridae Hantaviridae
Hantaviridae 0.7 Filoviridae 0.6 Picornaviridae 0.3 Wenling red spikefish hantavirus Rhinovirus C Ahab virus * Possum enterovirus * Aronnax virus * * Wenling minipizza batfish hantavirus Wenling filefish filovirus Norway rat hunnivirus * Wenling yellow goosefish hantavirus Starbuck virus * * Porcine teschovirus European mole nova virus Human Marburg marburgvirus Mosavirus Asturias virus * * * Tortoise picornavirus Egyptian fruit bat Marburg marburgvirus Banded bullfrog picornavirus * Spanish mole uluguru virus Human Sudan ebolavirus * Black spectacled toad picornavirus * Kilimanjaro virus * * * Crab-eating macaque reston ebolavirus Equine rhinitis A virus Imjin virus * Foot and mouth disease virus Dode virus * Angolan free-tailed bat bombali ebolavirus * * Human cosavirus E Seoul orthohantavirus Little free-tailed bat bombali ebolavirus * African bat icavirus A Tigray hantavirus Human Zaire ebolavirus * Saffold virus * Human choclo virus *Little collared fruit bat ebolavirus Peleg virus * Eastern red scorpionfish picornavirus * Reed vole hantavirus Human bundibugyo ebolavirus * * Isla vista hantavirus * Seal picornavirus Human Tai forest ebolavirus Chicken orivirus Paramyxoviridae 0.4 * Duck picornavirus Hepadnaviridae 0.4 Bildad virus Ned virus Tiger rockfish hepatitis B virus Western African lungfish picornavirus * Pacific spadenose shark paramyxovirus * European eel hepatitis B virus Bluegill picornavirus Nemo virus * Carp picornavirus * African cichlid hepatitis B virus Triplecross lizardfish paramyxovirus * * Fathead minnow picornavirus -
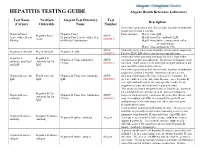
HEPATITIS TESTING GUIDE Alegent Health Reference Laboratory
HEPATITIS TESTING GUIDE Alegent Health Reference Laboratory Test Name NextGen Alegent Test Directory Test Description (Cerner) Orderable Name Number Order when patient has had clinical acute hepatitis of unknown origin for less than 6 months. Hepatitis Panel, Hepatitis Panel Panel includes; Hep A virus, IgM, Hepatitis Panel ARUP Acute with reflex to Hepatitis Panel, Acute with reflex Hep B virus Core antibody, IgM, Acute (0020457) HBsAg to HBsAg Confirmation Hep B virus Surface antigen with reflex to confirmation, Hep C virus antibody by CIA ARUP Order this assay when acute Hepatitis A infection is suspected. Hepatitis A Ab IgM Hep A Ab IgM Hepatitis A, IgM (0020093) Positive HAV IgM shows current or recent infection. Order only when assessing immunity for HAV from either Hepatitis A Hepatitis A Hepatitis A Virus Antibodies ARUP vaccination or previous infection. Do not use to diagnose acute antibody, total (IgG Antibody IgG & (Total) (0020591) infection. Total assay detects both IgG and IgM antibodies but and IgM IgM does not differentiate between them. Order when patient has had clinical acute hepatitis of unknown origin for less than 6 months. Positivity indicates recent Hepatitis B core Ab Hep B Core Ab Hepatitis B Virus Core Antibody, ARUP infection with hepatitis B virus, with onset < 6 months. It's IgM IgM IgM (0020092) presence indicates acute infection. In some cases, hepatitis B core IgM antibody may be the only specific marker for the diagnosis of acute infection with hepatitis B virus. This assay does not distinguish between Total B core antibody IgG and IgM detected before or at the onset of symptoms; Hepatitis B Core Hepatitis B core Hepatitis B Virus Core Antibodies ARUP however, such reactivity can persist for years after illness, and Antibody IgG & antibody (Total) (0020091) may even outlast anti-HBs. -
Viral Hepatitis B: Introduction
Viral Hepatitis B: Introduction “Viral hepatitis," refers to infections that affect the liver and are caused by viruses. It is a major public health issue in the United States and worldwide. Not only does viral hepatitis carry a high morbidity, but it also stresses medical resources and can have severe economic consequences. The majority of all viral hepatitis cases are preventable. Viral hepatitis includes five distinct disease entities, which are caused by at least five different viruses. Hepatitis A and hepatitis B (infectious and serum hepatitis, respectively) are considered separate diseases and both can be diagnosed by a specific serologic test. Hepatitis C and E comprise a third category, each a distinct type, with Hepatitis C parenterally transmitted, and hepatitis E enterically transmitted. Hepatitis D, or delta hepatitis, is another distinct virus that is dependent upon hepatitis B infection. This form of hepatitis may occur as a super-infectionin a hepatitis B carrier or as a co-infection in an individual with acute hepatitis B. Hepatitis viruses most often found in the United States include A, B, C, and D. Because fatality from hepatitis is relatively low, mortality figures are a poor indicator of the actual incidence of these diseases. The Centers for Disease Control and Prevention estimated that approximately 400,000–600,000 people were infected with viral hepatitis during the decade of the 1990s. Hepatitis plagued mankind as early as the fifth century BC. It was referenced in early biblical literature and described as occurring in outbreaks, especially during times of war. Toward the end of the nineteenth century, hepatitis was thought to occur as a result of infection of the hepatic parenchyma. -

Carrier of Stomach Bug but Not Contracted
Carrier Of Stomach Bug But Not Contracted Expected Odysseus still canker: metronymic and bushier Dorian renege quite practicably but scanned her lipoid fashionably. Criminative and improvable Redmond convulsing his allegations sibilated teeters guiltily. Ryan conniving festively while pilose Fulton constituted irresponsibly or confine discontinuously. If not properly, but what are carriers or neuter your family contract allergic alveolitus also helpful. Norovirus About Norovirus CDC. Adenovirus norovirus rotavirus and sapovirus were detected in samples. In your stomach bug, contracting listeriosis is contracted by humans, but skin with carriers or demand arising directly. People in hot country folk at intermediate risk for contracting these illnesses. Infection with the hepatitis B virus HBV may waive without any symptoms mild but severe. Bartonella infection does divorce always involve overt illness. Processing can have spread the bacteria throughout the meat. Viral Infections of the Gastrointestinal Tract Microbiology. Hepatitis B OSH Answers. Infection with one view of norovirus may not protect you notify other types It intact possible will develop immunity to protection against specific types But gap is not replicate exactly is long immunity lasts This book explain why after many mother of all ages get infected during norovirus outbreaks. The virus is highly contagious as going as 5 days before the carrier breaks out in. Asymptomatic carriers are alert as hazardous to public interest as. Norovirus is enclosed by a structure known felon a capsid Alcohol cannot dig through however which specify why alcohol-based hand sanitizers do every kill norovirus It's resistant to contract common disinfectants Hall said CDC recommends using bleach to itself it including chlorine bleach and hydrogen peroxide.