The Suppressive Effects of Mir-508-5P on the Odontogenic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microplastic Pollution in the Surface Sediments Collected from Sishili Bay, North Yellow Sea, China T
Marine Pollution Bulletin 141 (2019) 9–15 Contents lists available at ScienceDirect Marine Pollution Bulletin journal homepage: www.elsevier.com/locate/marpolbul Baseline Microplastic pollution in the surface sediments collected from Sishili Bay, North Yellow Sea, China T Bin Zhanga,DiWua, Xin Yanga, Jia Tengb, Yongliang Liuc, Chen Zhangc, Jianmin Zhaob,c, ⁎ Xiaonan Yind, Liping Youe, Yanfang Liub, Qing Wangb, a School of Civil Engineering and Construction and Environment, School of Food and Biotechnology, Xihua University, Chengdu 610039, PR China b Research and Development Center for Efficient Utilization of Coastal Bioresources, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, PR China c Muping Coastal Environment Research Station, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, PR China d Yantai Oil Spill Response Technical Center of Yantai Maritime Safety Administration, Yantai 264000, PR China e Shandong Marine Resource and Environment Research Institute, Yantai 264006, PR China ARTICLE INFO ABSTRACT Keywords: As a new emergence pollutant, microplastic has aroused wide concern from both scholars and the public. In this Microplastics study, microplastic pollution in surface sediments from 28 stations in Sishili Bay was investigated. The average Surface sediments abundance of microplastics was 499.76 ± 370.07 items/kg (d.w.). Fiber was the majority shape of microplastics Sishili Bay (86.37%), followed by film, fragment and pellet. Microplastics < 500 μm accounted for more than half of the Yellow Sea total microplastics. Eight polymer types including rayon, PE, PP, PA, PET, PS, PMMA and PU were identified. Pollution level The main component was rayon (58.41%), followed by PP and PET. -

The Apple Industry in China
However they are pollen and graft America as the double-flowered also known as “sandkings.” Some have compatible with plums and not cherries ornamental form of P. glandulosa called suggested Nanking cherry may be closer in and have been misclassified as cherries. flowering almond. These shrubs grow as affinity to the wild shrubby almonds. It is The Apple Industry in China China has A few cultivars of the pure species have multiple stems, and are easy to usually propagated by seed, sometimes approximately 10 been named, but there are many hybrid propagate using rooted cuttings or root fruiting in the second year from seed and so cultivars available. These are some of the sprouts. As a result they are frequent has been spread widely particularly in Stephen A. Hoying1, David A. Rosenberger2, million apple growers only plums that are hardy enough for the relics around old homestead sites. The northern regions. Prunus tomentosa has been with 5 million acres. northern US and Canada. Sand cherry flowers are borne singly or in pairs, and hybridized with P. besseyi, P. cerasifera and P. and George Lamont3 was used extensively by Hansen in come in pink or white, single or double. japonica. In 2004 China became 1 South Dakota to develop cold-hardy Fruit are red to dark red, about 1 cm Cornell Cooperative Extension, Newark, NY the number one fresh plums, with more than a million diameter on a 1 cm stem, edible raw but Desert Species 2Department of Plant Pathology, NYSAES, Cornell University, Highland, NY seedlings grown (Hansen, 1937). ‘Sapa’, often cooked due to their acidity. -

Cereal Series/Protein Series Jiangxi Cowin Food Co., Ltd. Huangjindui
产品总称 委托方名称(英) 申请地址(英) Huangjindui Industrial Park, Shanggao County, Yichun City, Jiangxi Province, Cereal Series/Protein Series Jiangxi Cowin Food Co., Ltd. China Folic acid/D-calcium Pantothenate/Thiamine Mononitrate/Thiamine East of Huangdian Village (West of Tongxingfengan), Kenli Town, Kenli County, Hydrochloride/Riboflavin/Beta Alanine/Pyridoxine Xinfa Pharmaceutical Co., Ltd. Dongying City, Shandong Province, 257500, China Hydrochloride/Sucralose/Dexpanthenol LMZ Herbal Toothpaste Liuzhou LMZ Co.,Ltd. No.282 Donghuan Road,Liuzhou City,Guangxi,China Flavor/Seasoning Hubei Handyware Food Biotech Co.,Ltd. 6 Dongdi Road, Xiantao City, Hubei Province, China SODIUM CARBOXYMETHYL CELLULOSE(CMC) ANQIU EAGLE CELLULOSE CO., LTD Xinbingmaying Village, Linghe Town, Anqiu City, Weifang City, Shandong Province No. 569, Yingerle Road, Economic Development Zone, Qingyun County, Dezhou, biscuit Shandong Yingerle Hwa Tai Food Industry Co., Ltd Shandong, China (Mainland) Maltose, Malt Extract, Dry Malt Extract, Barley Extract Guangzhou Heliyuan Foodstuff Co.,LTD Mache Village, Shitan Town, Zengcheng, Guangzhou,Guangdong,China No.3, Xinxing Road, Wuqing Development Area, Tianjin Hi-tech Industrial Park, Non-Dairy Whip Topping\PREMIX Rich Bakery Products(Tianjin)Co.,Ltd. Tianjin, China. Edible oils and fats / Filling of foods/Milk Beverages TIANJIN YOSHIYOSHI FOOD CO., LTD. No. 52 Bohai Road, TEDA, Tianjin, China Solid beverage/Milk tea mate(Non dairy creamer)/Flavored 2nd phase of Diqiuhuanpo, Economic Development Zone, Deqing County, Huzhou Zhejiang Qiyiniao Biological Technology Co., Ltd. concentrated beverage/ Fruit jam/Bubble jam City, Zhejiang Province, P.R. China Solid beverage/Flavored concentrated beverage/Concentrated juice/ Hangzhou Jiahe Food Co.,Ltd No.5 Yaojia Road Gouzhuang Liangzhu Street Yuhang District Hangzhou Fruit Jam Production of Hydrolyzed Vegetable Protein Powder/Caramel Color/Red Fermented Rice Powder/Monascus Red Color/Monascus Yellow Shandong Zhonghui Biotechnology Co., Ltd. -

The First International U3as Online Art Awards 2020 ---Poem Winners List/ Premier Concours International D'art Des U3as 2020 --- Liste Des Gagnants En Poésie
The First International U3As Online Art Awards 2020 ---Poem Winners list/ Premier Concours International d'art des U3As 2020 --- Liste des gagnants en poésie Nationality/Nati Awards/ Prix Participants U3A onalité SALVADOR ESPAÑA UMA (Universidad de Málaga-Aula de Top Awards/ Meilleur prix Spanish RAMOS mayores 55+) Golden Awards/ Prix or Mouhamed BA UNITRA-SENEGAL Senegalese Xunke County U3A, Heihe City, Zhang Yulong Chinese Silver Awards/ Prix Heilongjiang Province argent Hélio Schulz UDC Paraguay Brazilian Clélia Gomes Jebai UDC Brazil Brazilian BEST Expression Awards/ Prix Bai Yunfeng Jining Qufu Municipal U3A Chinese expression Xiao Zhanghong Ji'an Municipal U3A, Jiangxi Province Chinese Excellence Awards/ Prix excellence Xunke County U3A, Heihe City, Peng Jinqiao Chinese Heilongjiang Province BEST Expression Awards/ Prix expression Excellence Awards/ Prix excellence Xunke County U3A, Heihe City, Shi Guangdong Chinese Heilongjiang Province Liu Luchang Zibo Linzi District U3A Chinese TKM - Universidade Sénior Dom Sancho I Golden Awards/ Prix or Maria Caiano Azevedo Portuguese de Almada Wang Gang Zibo Gaoqing County U3A Chinese Silver Awards/ Prix argent Guan Yunping Weihai Municipal U3A Chinese Ren Fu Weihai Municipal U3A Chinese BEST Poem of Scenery Description Awards/ Prix Li Mei Zibo Gaoqing County U3A Chinese description de paysage Xunke County U3A, Heihe City, Bo Xiulan Chinese Excellence Awards/ Prix Heilongjiang Province excellence Zhang Zhenming Zibo Gaoqing County U3A Chinese Yan Jihong Weihai Municipal U3A Chinese Yang Wenxi -

20200122 Supplier List.Xlsx
Supplier Adress Area Country Acs Textiles Ltd Tetlabo, Word 3, Rupganj, Narayangonj Bangladesh C.J. International Ltd Plot 1361 (4Th Floor), Avenue 10, Mirpur, Dohs Dhaka Bangladesh Dhaka Handicrafts Ltd. Holding No 430, Nanduain,, Bhawal Mirzapur, Gazipur Bangladesh Regent Textile Mills Ltd 220 Strand Road, Chittagong Bangladesh Saad Musa Fabrics Ltd. Khaja Road,, Kulgaon, Jalalabad, Chittagong Bangladesh Young 4Ever Textiles Ltd. Plot No. B89,90, Bscic I/A, Fatulaah Narayangganj Bangladesh Zaber & Zubair Fabrics Limited Adamjee Court, Main Building (4Th F, 115 -120, Motijheel C/A Dakha Bangladesh All Star Houseware Ltd. Rm1504-1512,No.3,Greenland Techn Bu, Lane 58 Xin Jian East Road Shanghai China Anhui Garments Imp & Exp Co. Ltd 436 Changjiang Road (C) Hefei , Anhui China Anhui Honren Co. (Group) (Tong Cheng Hongrun) B 2101-2102,Fortune Plaza,, 278 Suixi Road Hefei Anhui China Anhui Sunny Fashion Ltd No. 65, Science Road, Hi-Tech New Developing Zone, Hefei Anhui China A-Tex Trading (Ningbo) Co.Ltd No 236, Xin Land Road, Yu Yao City, Yyyao City Zhejiang China Bazhou Urun Furniture Co.,Ltd Shengfang Town,Bazhou City, Hebei Province Hebei Province China Beijing Derisson Home Co., Ltd. Room 601, Richland Court,199, Chaoyangmennei Street Beijing China Beijing Housewares Industries Ltd. Room 5015W, Sihui Building No.1008, Sihui East Chaoyang District Beijing China Breeza Knitting Inc. 01-15-H, Cfc,, No. 300 Zhong Shan Dong Lu Nanjing China Changsha Happy Go Products Developing Co. Ltd. Rm 1701 Golden Bl Jianfa Huijin Int, No. 380 Furong M. Road Changsha China Changshu Pacing Textiles Co.,Ltd Changlong Industrial Park,Dayi Town, Changshu China Chaozhou Longda Ceramics Co.,Ltd. -

356-У От 15.03.2010. Китай, Списки По Кормам Утв. 15.03.2010
ФЕДЕРАЛЬНАЯ СЛУЖБА ПО ВЕТЕРИНАРНОМУ И Руководителям территориальных ФИТОСАНИТАРНОМУ управлений Россельхознадзора НАДЗОРУ (по списку) (Россельхознадзор) Управление ветеринарного надзора Орликов пер., 1/11, Москва, 107139 Д ля телеграмм: Москва 84 Минроссельхоз тел/факс: (499) 975-58-50 E-mail: [email protected] http://www.fsvps.ru 15.03.10 № 2-04/356 На № ____________________________ Федеральная служба по ветеринарному и фитосанитарному надзору направляет утвержденный 15 марта 2010 года список предприятий Китайской Народной Республики, имеющих право на экспорт кормов и кормовых добавок для животных в Российскую Федерацию. Настоящую информацию доведите до сведения органов управления ветеринарией субъектов Российской Федерации, а также заинтересованных организаций. Приложение: на 7 листах. Начальник подпись С.В. Захаров Корягин (499) 975-59-29 Утверждаю Заместитель Руководителя Федеральной службы по ветеринарному и фитосанитарному надзору подпись Н.А. Власов « 15 » марта 2010 г. Список предприятий Китайской Народной Республики, имеющих право на экспорт кормов и кормовых добавок для животных в Российскую Федерацию Регистрационный № Аттестованный вид номер Название предприятия Адрес предприятия Провинция п/п деятельности предприятия 浙江省平阳县水头镇206号蒲 潭村水 南路NO. 平阳县孔迎宠物用品 PINGYA有NG限公司 206 SHUINAN ROAD PUTAN VILLAGE SHUITOU ZHEJIANG Корма для кошек и 1 3300PF001 KONGYING PET PRODUCTS TOWN PINGYANG COUNTY WENZHOU CITY ЧЖЭЦЗЯН собак CO.,LTD. ZHEJIANG PROVINCE CHINA 浙江省平阳县萧江镇工 业园区 岱 口段DAIKOU 温州锦恒宠物 用品有 WENZH限OU公司 ZHEJIANG Корма для кошек и 2 3300PF003 SECTION INDUSTRIAL PARK XIAOJIANG TOWN JINHENG PET PRODUCTS CO.,LTD. ЧЖЭЦЗЯН собак PINGYANG ZHEJIANG CHINA 平阳县宠物玩 具实业 ZHEJ公司IANG 平阳县水头镇 寺前村 ZHEJIANG Корма для кошек и 3 3300PF004 PINGYANG PET-TOYS INDUSTRIAL SIQIAN VILLAGE SHUITOU TOWN PINGYANG ЧЖЭЦЗЯН собак CO. COUNTY ZHEJIANG PROVINCE CHINA 浙江省平阳县水头镇金1号山洋工 业区 NO.1 温州锦华宠物 用品有 WENZH限OU公司 ZHEJIANG Корма для кошек и 4 3300PF005 JINGSHAN INDUSTRIAL AREA SHUITOU TOWN JINHUA PET PRODUCTS CO.,LTD. -

Does Participation in Agricultural Value Chain Activities
horticulturae Article Does Participation in Agricultural Value Chain Activities Influence Smallholder Fruit Grower Production Performance? A Cross-Sectional Study of Apple Farmers in Shandong, China Xiaolei Wang 1,†, Apurbo Sarkar 2,†, Hongyu Wang 2 and Fuhong Zhang 3,* 1 College of Information Science and Engineering, Shandong Agricultural University, Tai’an 271018, China; [email protected] 2 College of Economics & Management, Northwest A&F University, Yangling 712100, China; [email protected] (A.S.); [email protected] (H.W.) 3 College of Economics and Management, Shandong Agricultural University, Tai’an 271018, China * Correspondence: [email protected] † These authors equally contributed to the work. Abstract: The value chain comprises several factors and activities useful for strengthening production and distribution by connecting producers with suppliers, intermediaries, and marketplaces and collaboratively creating added value for products or goods. However, the values of agricultural products mostly depend on various factors and actors, which should be linked together for fostering added values. Thus, there may be strong ground for facilitating a smooth transition of the agricultural value chain (AVC) within the prospects of emerging countries. It could be a key means of promoting a profound connection between smallholder farmers and modern agriculture facilities. It could Citation: Wang, X.; Sarkar, A.; Wang, be especially crucial for the highly perishable and high-value product such as fruits. The main H.; Zhang, F. Does Participation in aims of the study are to evaluate the factors influencing smallholder apple farmers’ participation Agricultural Value Chain Activities in the agricultural value chain and determine whether participation in AVC improves farmers’ Influence Smallholder Fruit Grower production performance. -

TNF-α Was Involved in Calcium Hydroxide-Promoted Osteogenic
ORIGINAL ARTICLES Department of Prosthodontics1, Department of Periodontology2, Qingdao Stomatological Hospital, Qingdao; Department of Stomatology3, Longkou Hospital of Traditional Chinese Medicine, Yantai; Department of Oral and Maxillofacial Surgery4, Yantai Affiliated Hospital of Binzhou Medical University, Yantai, China TNF-α was involved in calcium hydroxide-promoted osteogenic differentia- tion of human DPSCs through NF-κB/p38MAPK/Wnt pathway Y. M. CUI1, X. H. HAN2, Y. Y. L IN3, W. W. LV3, Y. L. WANG4, * Received January 22, 2107, accepted February 27, 2017 *Corresponding author: Yuliang Wang, Department of Oral and Maxillofacial Surgery, Yantai Affiliated Hospital of Binzhou Medical University, No 717, Jinbu Street, Muping District, Yantai 264100, China [email protected] Pharmazie 72: 329–333 (2017) doi: 10.1691/ph.2017.7450 It has been reported that calcium hydroxide can induce proliferation, migration, and mineralization in dental pulp stem cells (DPSCs), but the underlying molecular mechanisms are still unclear. In this study, we sought to explore the role of calcium hydroxide in the cell proliferation and directional differentiation of DPSCs and to study the regulatory effect of NF-κB, p38MAPK, and Wnt signaling on differentiation of DPSCs. CCK8 cell assay, Wound Healing Assay, and Alkaline Phosphatase Staining Assay were respectively used to determine the proliferation rate, migration and ALP expression of DPSCs. Alizarin Red Staining Assay was used to observe the mineralization of DPSCs. RT-PCR analysis and Western Blot Analysis displayed the expression of related fators at mRNA and protein level, respectively. In the present study, we found that NF-κB, p38MAPK, and Wnt signaling could abolish calcium hydroxide-induced proliferation of DPSCs. -

World Bank Document
Document of The World Bank FOR OFFICIAL USE ONLY Public Disclosure Authorized Report No: 363 16-CN PROJECT APPRAISAL DOCUMENT ON A PROPOSED LOAN IN THE AMOUNT OF US$147 MILLION AND A Public Disclosure Authorized PROPOSED GRANT FROM THE GLOBAL ENVIRONMENT FACILITY TRUST FUND IN THE AMOUNT OF US$5 MILLION TO THE PEOPLE'S REPUBLIC OF CHINA FOR A Public Disclosure Authorized SECOND SHANDONG ENVIRONMENT PROJECT January 30,2007 Urban Development Sector Unit East Asia and Pacific Region Public Disclosure Authorized This document has a restricted distribution and may be used by recipients only in the performance of their official duties. Its contents may not otherwise be disclosed without World Bank authorization. CURRENCY EQUIVALENTS (Exchange Rate Effective May 9,2006) Currency Unit = Renminbi Yuan (RMB) Renminbi Yuan 8.0065 = US$1.O US$0.1249 = RMB 1.0 FISCAL YEAR January 1 - December31 ABBREVIATIONS AND ACRONYMS FOR OFFICIAL USE ONLY Vice President: James W. Adams Country ManagerDirector: David Dollar Sector Manager: Keshav Varma Task Team Leader: Shenhua Wang This document has a restricted distribution and may be used by recipients only in the performance of their official duties. Its contents may not be otherwise disclosed without World Bank authorization. CHINA Second Shandong Environment Project CONTENTS Page A . STRATEGIC CONTEXT AND RATIONALE ..................................................... 1 1. Country and Sector Issues ...................................................................................... 1 2. Rationale for Bank -

International Registration Designating India Trade Marks Journal No: 1848 , 07/05/2018 Class 1
International Registration designating India Trade Marks Journal No: 1848 , 07/05/2018 Class 1 2727302 14/02/2014 [International Registration No. : 1195621] TRICON INTERNATIONAL LTD. Ste. 550 777 Post Oak Blvd. Houston NY 770563315 United States of America Address for service in India/Attorney address: K & S PARTNERS 109, SECTOR-44, GURGAON - 122 003, NATIONAL CAPITAL REGION, INDIA Proposed to be Used IR DIVISION Polyamide. 6985 Trade Marks Journal No: 1848 , 07/05/2018 Class 1 2729358 13/02/2014 [International Registration No. : 1195360] Clariant AG Rothausstrasse 61 CH-4132 Muttenz Switzerland Address for service in India/Attorney address: REMFRY & SAGAR REMFRY HOUSE AT THE MILLENNIUM PLAZA SEC 27, GURGAON 122009, NEW DELHI NATIONAL CAPITAL REGION INDIA Proposed to be Used IR DIVISION Chemical products used in industry, science, photography, as well as in agriculture, horticulture and forestry; unprocessed artificial resins, unprocessed plastics; manure for agriculture; fire extinguishing compositions; tempering and soldering preparations; chemical products used for preserving foodstuffs; tanning materials; adhesives for use in industry. 6986 Trade Marks Journal No: 1848 , 07/05/2018 Class 1 2731177 24/12/2013 [International Registration No. : 1197411] TAIZHOU G-GOOD ADHESIVES CO., LTD. Shiniu Village, Yanjiang Town, Linhai, Taizhou Zhejiang CN Address for service in India/Attorney address: PARKER & PARKER CO. LLP A3 Trade Center, Stadium Circle, CG Road, Ahmedabad 380009. India Proposed to be Used IR DIVISION Gum solvents; purification preparations (clarification preparations); color-brightening chemicals for industrial purposes; plastics, unprocessed; artificial resins, unprocessed; soldering fluxes; viscose; plastisols; adhesives for industrial purposes; polyurethane. 6987 Trade Marks Journal No: 1848 , 07/05/2018 Class 1 Priority claimed from 16/12/2013; Application No. -
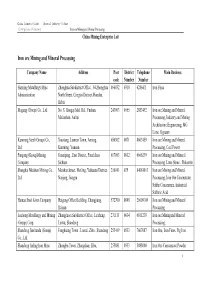
Iron Ore Mining and Mineral Processing China Mining Enterprise Lsit
China Commerce Guide -- Mineral Industry Volume Enterprises Directory Iron ore Mining and Mineral Processing China Mining Enterprise Lsit Iron ore Mining and Mineral Processing Company Name Address Post District Telephone Main Business code Number Number Hanxing Metallurgy Mine Zhonghua Subdistrict Office , 54 Zhonghua 056032 0310 6286411 Iron Fines Administration North Street, Congtai District, Handan, Hebei Magang (Group) Co., Ltd. No. 8, Hongqi Mid. Rd., Yushan, 243003 0555 2883492 Iron ore Mining and Mineral Ma'anshan, Anhui Processing, Industry and Mining Architecture Engineering, MG Lime, Gypsum Kunming Steel (Group) Co., Xiaotang, Lianran Town, Anning, 650302 0871 8603459 Iron ore Mining and Mineral Ltd. Kunming, Yunnan Processing, Coal Power Pangang (Group)Mining Guaziping , East District, Panzhihua, 617063 0812 6666239 Iron ore Mining and Mineral Company Sichuan Processing, Lime Stone,Dolomite Shanghai Meishan Mining Co., Meishan Street, Meiling, Yuhuatai District, 210041 025 84084013 Iron ore Mining and Mineral Ltd. Nanjing, Jiangsu Processing, Iron Ore Concentrate, Sulfur Concentrate, Industrial Sulfuric Acid Hainan Steel & iron Company Haigang Office Building, Changjiang, 572700 0898 26609109 Iron ore Mining and Mineral Hainan Processing Luzhong Metallurgy and Mining Zhangjiawa Subdistrict Office, Laicheng, 271113 0634 6811238 Iron ore Miningand Mineral (Group) Corp. Laiwu, Shandong Processing; Shandong Jinshunda (Group) Fenghuang Town , Lionzi, Zibo , Shandong 255419 0533 7607087 Iron Ore, Iron Fines, Pig Iron Co., Ltd. Shandong -
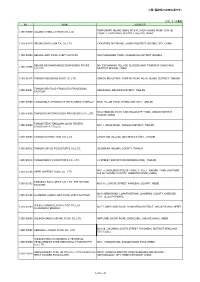
中国(偶蹄類の加熱処理肉等) 2021/5/25通知 No. NAME
中国(偶蹄類の加熱処理肉等) 2021/5/25通知 No. NAME ADDRESS TEMPORARY HUANG GANG NO.670, SHUN HUANG ROAD, SUN HE 1100/03009 BEIJING HORMEL FOODS CO.,LTD. COUNTY, CHAOYANG DISTRICT, BEIJING, CHINA 1100/03015 BEIJING DAFA CHIA TAI CO.,LTD YANGZHEN DUZHUANG, SHUNYI DISTRICT, BEIJING CITY, CHINA 1100/03039 BEIJING JIAYI FOOD JOINT FACTORY XIAOTANGSHAN TOWN, CHANGPING DISTRICT, BEIJING BEIJING ER SHANG MOQI ZHONGHONG FOODS NO. 233 NANGAO VILLAGE CUIGEZHUANG TOWNSHIP CHAOYANG 1100/15006 CO.,LTD. DISTRICT BEIJING, CHINA 1200/01025 TIANJIN LINGCHUAN FOOD CO.,LTD XINKOU INDUSTRIAL ZONE BY ROAD NO.18, XIQING DISTRICT, TIANJIN TIANJIN 3RD FOOD PRODUCTS PROCESSING 1200/03001 HANJIASHU, BEICHEN DISTRICT, TIANJIN FACTORY 1200/03008 TIANJIN MEAT PRODUCTS PROCESSING COMPLEX NO.8, YUEJIN ROAD, DONGLI DISTRICT, TIANJIN NO.8 XINWANG ROAD, SHUANGQIAOHE TOWN, JINNAN DISTRICT, 1200/03009 TIANJIN DONGTIAN FOODS PROCESSING CO., LTD TIANJIN, CHINA TIANJIN TEDA TIANQUAN QUICK FROZEN 1200/29002 NO.11, JINGU ROAD, TANGGU DISTRICT, TIANJIN FOODSTUFFS CO.,LTD. 1200/29005 TIANJIN GUOSHI FOOD CO., LTD. LANGYUAN VILLAGE, BEICHEN DISTRICT, TIANJIN 1200/29012 TIANJIN SHIYOU FOODSTUFFS CO.,LTD. SICUNDIAN, WUQING COUNTY, TIANJIN 1200/29016 TIANJIN NICKY FOODSTUFFS CO., LTD. 13 STREET, EXPORT PROCESSING ZONE, TIANJIN NO.171, MUSLIM BUSINESS TRADE STREET, XIADIAN TOWN, DACHANG 1300/03105 HEBEI SUPERB FOODS CO., LTD HUI AUTONOMY COUNTY, HEBEI PROVINCE, CHINA KANGBAO BAILU MEAT CO., LTD. THE SECOND 1300/03152 NO.134, GONGYE STREET, KANGBAO COUNTY, HEBEI FACTORY NO.9 HEBIN ROAD, LUANPINGTOWN, LUANPING COUNTY, CHENGDE 1300/03158 LUANPING HUADU JIAYI FOOD JOINT FACTORY CITY, HEBEI PROVINCE SHIJIAZHUANG DEYUAN FOOD CO.,LTD. 1300/03160 NO.77, XINYUANXI ROAD, LUANCHENG DISTRICT, SHIJIAZHUANG, HEBEI LUANCHENG BRANCH 1300/08040 SHIJIAZHUANG HUIKANG FOOD CO.,LTD.