Dynamics of Tree Diversity in Undisturbed and Logged Subtropical Rainforest in Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Native Vegetation of the Nattai and Bargo Reserves
The Native Vegetation of the Nattai and Bargo Reserves Project funded under the Central Directorate Parks and Wildlife Division Biodiversity Data Priorities Program Conservation Assessment and Data Unit Conservation Programs and Planning Branch, Metropolitan Environmental Protection and Regulation Division Department of Environment and Conservation ACKNOWLEDGMENTS CADU (Central) Manager Special thanks to: Julie Ravallion Nattai NP Area staff for providing general assistance as well as their knowledge of the CADU (Central) Bioregional Data Group area, especially: Raf Pedroza and Adrian Coordinator Johnstone. Daniel Connolly Citation CADU (Central) Flora Project Officer DEC (2004) The Native Vegetation of the Nattai Nathan Kearnes and Bargo Reserves. Unpublished Report. Department of Environment and Conservation, CADU (Central) GIS, Data Management and Hurstville. Database Coordinator This report was funded by the Central Peter Ewin Directorate Parks and Wildlife Division, Biodiversity Survey Priorities Program. Logistics and Survey Planning All photographs are held by DEC. To obtain a Nathan Kearnes copy please contact the Bioregional Data Group Coordinator, DEC Hurstville Field Surveyors David Thomas Cover Photos Teresa James Nathan Kearnes Feature Photo (Daniel Connolly) Daniel Connolly White-striped Freetail-bat (Michael Todd), Rock Peter Ewin Plate-Heath Mallee (DEC) Black Crevice-skink (David O’Connor) Aerial Photo Interpretation Tall Moist Blue Gum Forest (DEC) Ian Roberts (Nattai and Bargo, this report; Rainforest (DEC) Woronora, 2003; Western Sydney, 1999) Short-beaked Echidna (D. O’Connor) Bob Wilson (Warragamba, 2003) Grey Gum (Daniel Connolly) Pintech (Pty Ltd) Red-crowned Toadlet (Dave Hunter) Data Analysis ISBN 07313 6851 7 Nathan Kearnes Daniel Connolly Report Writing and Map Production Nathan Kearnes Daniel Connolly EXECUTIVE SUMMARY This report describes the distribution and composition of the native vegetation within and immediately surrounding Nattai National Park, Nattai State Conservation Area and Bargo State Conservation Area. -

NSW Rainforest Trees Part
This document has been scanned from hard-copy archives for research and study purposes. Please note not all information may be current. We have tried, in preparing this copy, to make the content accessible to the widest possible audience but in some cases we recognise that the automatic text recognition maybe inadequate and we apologise in advance for any inconvenience this may cause. · RESEARCH NOTE No. 35 ~.I~=1 FORESTRY COMMISSION OF N.S.W. RESEARCH NOTE No. 35 P)JBLISHED 197R N.S.W. RAINFOREST TREES PART VII FAMILIES: PROTEACEAE SANTALACEAE NYCTAGINACEAE GYROSTEMONACEAE ANNONACEAE EUPOMATIACEAE MONIMIACEAE AUTHOR A.G.FLOYD (Research Note No. 35) National Library of Australia card number and ISBN ISBN 0 7240 13997 ISSN 0085-3984 INTRODUCTION This is the seventh in a series ofresearch notes describing the rainforest trees of N.S. W. Previous publications are:- Research Note No. 3 (I 960)-N.S.W. Rainforest Trees. Part I Family LAURACEAE. A. G. Floyd and H. C. Hayes. Research Note No. 7 (1961)-N.S.W. Rainforest Trees. Part II Families Capparidaceae, Escalloniaceae, Pittosporaceae, Cunoniaceae, Davidsoniaceae. A. G. Floyd and H. C. Hayes. Research Note No. 28 (I 973)-N.S.W. Rainforest Trees. Part III Family Myrtaceae. A. G. Floyd. Research Note No. 29 (I 976)-N.S.W. Rainforest Trees. Part IV Family Rutaceae. A. G. Floyd. Research Note No. 32 (I977)-N.S.W. Rainforest Trees. Part V Families Sapindaceae, Akaniaceae. A. G. Floyd. Research Note No. 34 (1977)-N.S.W. Rainforest Trees. Part VI Families Podocarpaceae, Araucariaceae, Cupressaceae, Fagaceae, Ulmaceae, Moraceae, Urticaceae. -

TML Propagation Protocols
PROPAGATION PROTOCOLS This document is intended as a guide for Tamborine Mountain Landcare members who wish to assist our regeneration projects by growing some of the plants needed. It is a work in progress so if you have anything to add to the protocols – for example a different but successful way of propagating and growing a particular plant – then please give it to Julie Lake so she can add it to the document. The idea is that our shared knowledge and experience can become a valuable part of TML's intellectual property as well as a useful source of knowledge for members. As there are many hundreds of plants native to Tamborine Mountain, the protocols list will take a long time to complete, with growing information for each plant added alphabetically as time permits. While the list is being compiled by those members with competence in this field, any TML member with a query about propagating a particular plant can post it on the website for other me mb e r s to answer. To date, only protocols for trees and shrubs have been compiled. Vines and ferns will be added later. Fruiting times given are usual for the species but many rainforest plants flower and fruit opportunistically, according to weather and other conditions unknown to us, thus fruit can be produced at any time of year. Finally, if anyone would like a copy of the protocols, contact Julie on [email protected] and she’ll send you one. ………………….. Growing from seed This is the best method for most plants destined for regeneration projects for it is usually fast, easy and ensures genetic diversity in the regenerated landscape. -

PLANT COMMUNITY FIELD GUIDE Introduction to Rainforest
PLANT COMMUNITY FIELD GUIDE Introduction to Rainforest Communities Table of Contents (click to go to page) HCCREMS Mapping ....................................................................... 3 Field Data Sheet ............................................................................. 4 Which of the following descriptions best describes your site? ................................................................ 5 Which plant community is it? .......................................................... 9 Rainforest communities of the Lower Hunter .................................. 11 Common Rainforest Species of the Lower Hunter ........................................................................ 14 A picture guide to common rainforest species of the Lower Hunter ........................................................... 17 Weeding of Rainforest Remnants ................................................... 25 Rainforest Regeneration near Black Jacks Point ............................ 27 Protection of Rainforest Remnants in the Lower Hunter & the Re-establishment of Diverse, Indigenous Plant Communities ... 28 Guidelines for a rainforest remnant planting program ..................... 31 Threatened Species ....................................................................... 36 References ..................................................................................... 43 Acknowledgements......................................................................... 43 Image Credits ................................................................................ -
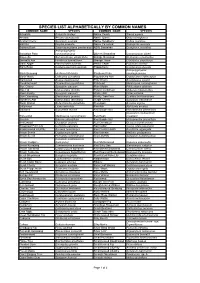
Species List Alphabetically by Common Names
SPECIES LIST ALPHABETICALLY BY COMMON NAMES COMMON NAME SPECIES COMMON NAME SPECIES Actephila Actephila lindleyi Native Peach Trema aspera Ancana Ancana stenopetala Native Quince Guioa semiglauca Austral Cherry Syzygium australe Native Raspberry Rubus rosifolius Ball Nut Floydia praealta Native Tamarind Diploglottis australis Banana Bush Tabernaemontana pandacaqui NSW Sassafras Doryphora sassafras Archontophoenix Bangalow Palm cunninghamiana Oliver's Sassafras Cinnamomum oliveri Bauerella Sarcomelicope simplicifolia Orange Boxwood Denhamia celastroides Bennetts Ash Flindersia bennettiana Orange Thorn Citriobatus pauciflorus Black Apple Planchonella australis Pencil Cedar Polyscias murrayi Black Bean Castanospermum australe Pepperberry Cryptocarya obovata Archontophoenix Black Booyong Heritiera trifoliolata Picabeen Palm cunninghamiana Black Wattle Callicoma serratifolia Pigeonberry Ash Cryptocarya erythroxylon Blackwood Acacia melanoxylon Pink Cherry Austrobuxus swainii Bleeding Heart Omalanthus populifolius Pinkheart Medicosma cunninghamii Blue Cherry Syzygium oleosum Plum Myrtle Pilidiostigma glabrum Blue Fig Elaeocarpus grandis Poison Corkwood Duboisia myoporoides Blue Lillypilly Syzygium oleosum Prickly Ash Orites excelsa Blue Quandong Elaeocarpus grandis Prickly Tree Fern Cyathea leichhardtiana Blueberry Ash Elaeocarpus reticulatus Purple Cherry Syzygium crebrinerve Blush Walnut Beilschmiedia obtusifolia Red Apple Acmena ingens Bollywood Litsea reticulata Red Ash Alphitonia excelsa Bolwarra Eupomatia laurina Red Bauple Nut Hicksbeachia -

Thysanoptera: Phlaeothripinae, Leeuweniini), with Comments on Related Old World Taxa
Blackwell Science, LtdOxford, UKAENAustralian Journal of Entomology1326-67562004 Australian Entomological SocietyMarch 20044312837Original ArticleAustralian long-tailed gall thripsLaurence A Mound Australian Journal of Entomology (2004) 43, 28–37 Australian long-tailed gall thrips (Thysanoptera: Phlaeothripinae, Leeuweniini), with comments on related Old World taxa Laurence A Mound CSIRO Entomology, GPO Box 1700, Canberra, ACT 2601, Australia. Abstract The Tribe Leeuweniini is a group of Old World Phlaeothripinae species that feed and usually induce irregular galls on the leaves of rainforest trees. These thrips all have the last abdominal segment unusually elongate, but this is a variable and homoplastic character state, and the tribe remains ill- defined. Worldwide, 27 species in three genera are now recognised, with five other generic names here included as synonyms of Leeuwenia Karny. From Australia, six species in two genera are recorded here occurring in the eastern rainforests. Four newly described Australian species and their host plants are: Leeuwenia diospyri sp. n. (Diospyros pentamera–Ebenaceae); L. polyosmae sp. n. (Polyosma cunninghamii–Grossulariaceae); L. scolopiae sp. n. (Scolopia braunii–Flacourtiaceae); and L. tetrastigmae sp. n. (Tetrastigma nitens–Vitaceae). The host association of L. convergens Hood is not known, but the sixth species, Neohoodiella jennibeardae Mound and Williams, breeds on two unrelated plants of which the leaves are similar in texture – Ficus coronata (Moraceae) and Rhipogonum elseyanum (Smilacaceae). Key words galls, Leeuwenia, Neohoodiella, rainforest trees. INTRODUCTION that was found living in an abandoned weevil mine on an Acacia phyllode in Queensland. In contrast to other insects, adults of the 3500 named species This paper gives some account of the six Australian mem- in the thysanopteran family Phlaeothripidae have the tenth bers of an Old World group of 27 described thrips species in abdominal segment forming a complete tube. -
Iden Tification G Uidelines for Endangered Ecolo Gical C
Supplementary Information for: Littoral Rainforest in the South East Corner, Sydney Basin and NSW North Coast bioregions Characteristic Species List Littoral Rainforest is characterised by the species listed in table below. As outlined in the Littoral Rainforest Identification Guideline, there are five recognised sub-alliances of Littoral Rainforest in NSW and there is considerable floristic variation between stands. A number of species characteristic of Littoral Rainforest in NSW reach their southern limits at various places along the coast. Details on species range and growth form are outlined in the table. The species present at any site will be influenced by the size of the site, recent rainfall or drought conditions and by its disturbance (including grazing, clearing and fire) history. Note that NOT ALL the species listed below need to be present at any one site for it to constitute Littoral Rainforest. General range Species name Common Name North from Trees (6m +) Widespread Acmena smithii Lilly Pilly Widespread Acronychia oblongifolia White Aspen Widespread Banksia integrifolia subsp. integrifolia Coastal Banksia Widespread Cryptocarya glaucescens Jackwood Widespread Cryptocarya microneura Murrogun Widespread Cyclophyllum longipetalum (formerly Coastal Canthium Canthium coprosmoides) Widespread Dendrocnide excelsa Giant Stinging Tree Widespread Ehretia acuminata Koda Widespread Elaeodendron australe (formerly Cassine Red Olive Berry australis) Widespread Eucalyptus tereticornis Forest Red Gum Widespread Ficus coronata Sanpaper Fig -

Australian Essential Oils History and Emerging Trends
Australian Essential Oils – History and Emerging Trends Ashley Dowell – Essential Oil Producers Association of Australia & Southern Cross University 23 rd October 2019 Introduction Australia has a unique flora largely of Gondwanan origin with later influence from the Indian subcontinent and South East Asia. Gondwana land was a pre-historic super continent including Australia, New Zealand, South America, Antarctica, Africa and the Indian sub-continent. Although separating early, it is proposed that Australia and India re-connected for a period of time before separating again. In more recent history Australia was connected to South-east Asia by land bridges which arose due to falling sea levels resulting from ice age glaciation Dominant plant families present in Australia include the essential oil bearing genus: Myrtaceae – Eucalyptus, Melaleuca, Leptospermum Rutaceae – Boronia, Citrus Other significant essential oil bearing plant families found in Australia are the Santalaceae, Myoporaceae, and Cupressaceae. Australia is often perceived as a land of deserts but is in fact a diverse collection of fire and drought adapted environments. Eucalypt woodlands make up to 39% of Australian vegetative cover, predominantly in New South Wales and Queensland followed by Hummock grasslands at 23% found mostly in Western Australia, Queensland and New SouthWales and Acacia woodlands occupying 10-20%, mostly in Western Australia, Queensland and New South Wales. There are significant areas of Acacia shrublands, Tussock grasslands and Chenopod shrubs and forblands, particularly in South Australia. There are relatively small (less than 70,000 Km2) but biologically significant areas of tropical and temperate rainforest in eastern coastal areas from Queensland to Tasmania, as well as Tall eucalypts forests and Callitris and Casuarina forests further inland and in sub-alpine areas. -

Farmaka 212 Volume 17 Nomor 3
Farmaka 212 Volume 17 Nomor 3 REVIEW ARTIKEL: AKTIVITAS FARMAKOLOGI DARI TANAMAN GENUS DYSOXYLUM Muhamad Nadiva Mardiana dan Raden Bayu Indradi Fakultas Farmasi Universitas Padjadjaran Jl. Raya Bandung Sumedang KM 21, Jatinangor, Sumedang, Jawa Barat 45363 [email protected], [email protected] Diserahkan 29/06/2019, diterima 23/01/2020 ABSTRAK Tanaman obat telah menjadi alternatif pengobatan pada masyarakat lokal untuk beberapa penyakit. Banyaknya resistensi mikroba terhadap obat kimia yang meningkat memunculkan beberapa penelitian untuk beralih ke obat herbal yang memiliki efek samping yang lebih sedikit dibandingkan obat kimia. Tanaman dari genus Dysoxylum sangat tersebar luas di benua Asia, dan ada kurang kebih 80 spesies yang sudah diuji, dan masih ada banyak spesies tanaman genus ini yang belum diuji aktivitas farmakologinya. Berdasarkan kebutuhan yang ada, pengkajian terhadap tanaman-tanaman genus Dysoxylum perlu dilakukan agar dapat memberikan manfaat secara menyeluruh terhadap pengembangan obat herbal. Dari hasil ulasan diperoleh beberapa tanaman genus ini, didapat hasil aktivitasnya yang berupa sitotoksik terhadap sel kanker payudara MCF-7, antiinflamasi, antibakteri, antifidan, antiplasmodium, antioksidan, sitoprotektif dan penghambat pertumbuhan bakteri dan jamur yang berasal dari tanaman Dysoxylum aborescens, Dysoxylum parasiticum, Dysoxylum lukii, Dysoxylum alliaceum, Dysoxylum hainanense, Dysoxylum caulostachyum, Dysoxylum gothadora, Dysoxylum binectariferum, Dysoxylum cauliflorum, Dysoxylum densiflorum. Kata -

Invasion and Management of a Woody Plant, Lantana Camara L., Alters Vegetation Diversity Within Wet Sclerophyll Forest in Southeastern Australia
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia Ben Gooden University of Wollongong, [email protected] Kris French University of Wollongong, [email protected] Peter J. Turner Department of Environment and Climate Change, NSW Follow this and additional works at: https://ro.uow.edu.au/scipapers Part of the Life Sciences Commons, Physical Sciences and Mathematics Commons, and the Social and Behavioral Sciences Commons Recommended Citation Gooden, Ben; French, Kris; and Turner, Peter J.: Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia 2009. https://ro.uow.edu.au/scipapers/4953 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia Abstract Plant invasions of natural communities are commonly associated with reduced species diversity and altered ecosystem structure and function. This study investigated the effects of invasion and management of the woody shrub Lantana camara (lantana) in wet sclerophyll forest on the south-east coast of Australia. The effects of L. camara invasion and management on resident vegetation diversity and recruitment were determined as well as if invader management initiated community recovery. Vascular plant species richness, abundance and composition were surveyed and compared across L. -

Vegetation and Flora of Booti Booti National Park and Yahoo Nature Reserve, Lower North Coast of New South Wales
645 Vegetation and flora of Booti Booti National Park and Yahoo Nature Reserve, lower North Coast of New South Wales. S.J. Griffith, R. Wilson and K. Maryott-Brown Griffith, S.J.1, Wilson, R.2 and Maryott-Brown, K.3 (1Division of Botany, School of Rural Science and Natural Resources, University of New England, Armidale NSW 2351; 216 Bourne Gardens, Bourne Street, Cook ACT 2614; 3Paynes Lane, Upper Lansdowne NSW 2430) 2000. Vegetation and flora of Booti Booti National Park and Yahoo Nature Reserve, lower North Coast of New South Wales. Cunninghamia 6(3): 645–715. The vegetation of Booti Booti National Park and Yahoo Nature Reserve on the lower North Coast of New South Wales has been classified and mapped from aerial photography at a scale of 1: 25 000. The plant communities so identified are described in terms of their composition and distribution within Booti Booti NP and Yahoo NR. The plant communities are also discussed in terms of their distribution elsewhere in south-eastern Australia, with particular emphasis given to the NSW North Coast where compatible vegetation mapping has been undertaken in many additional areas. Floristic relationships are also examined by numerical analysis of full-floristics and foliage cover data for 48 sites. A comprehensive list of vascular plant taxa is presented, and significant taxa are discussed. Management issues relating to the vegetation of the reserves are outlined. Introduction The study area Booti Booti National Park (1586 ha) and Yahoo Nature Reserve (48 ha) are situated on the lower North Coast of New South Wales (32°15'S 152°32'E), immediately south of Forster in the Great Lakes local government area (Fig. -

Antarctic Beech (Nothofagus Moorei)
Dandarrga Nursery Native Species Labels A - M Antarctic Beech (Nothofagus moorei) Nothofagaceae A Gondwana rainforest tree 25 – 50 m Flowers Nov - Dec, seed pods Dec - Feb Range: High altitude rainforest of eastern Australia. This tree can reach a great age. New growth is red, and the complex root structure can grow multiple trunks, adorned with epiphytic orchids, ferns, fungi, mosses, liverworts and lichens. Bamboo Grass (Austrostipa ramosissima) Poaceae Native grass up to 1 to 2.5 m tall, 1.5 m wide Flowers: year round Range: S.E NSW to N.E QLD Stout Bamboo Grass is a tall ornamental grass. Fast growing and long lived. Useful container or border plant or for erosion and weed control. Attracts birds and small reptiles. Hardy; frost, drought and damp tolerant and grows in most soil conditions. Can be cut back hard to rejuvenate. Grows best with full or partial sun in shelter. Banana Bush (Tabernaemontana pandacaqui) Apocynaceae Deciduous shrub or small tree 1.5-14m Flowers: White; spring/summer Range: Manning River NSW to Cooktown QLD Normally growing to 1.5-3m in cultivation and can be pruned. Dense understory shrub with pretty tubular scented flowers. Unusual orange/ yellow fruit resemble small bananas but are poisonous to eat. Normally suitable for pruning. Adaptable to a range of moist, well-drained soil and prefers full or part shade. Dandarrga Nursery Native Species Labels A - M Basket Grass (Lomandra longifolia labill) Asparagaceae Native grass up to 1.2 m high & over 1m wide Flowers: cream to yellow from late winter to summer. Grows in a range of habitats FIRE RETARDANT SPECIES.