Chromosome Numbers in Pandanus, Sparganium and Typhal in the Present Paper the Chromosome Numbers of Some Species of Pandanus
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field. -

The Fossil Record of Angiosperm Families in Relation to Baraminology
The Proceedings of the International Conference on Creationism Volume 7 Article 31 2013 The Fossil Record of Angiosperm Families in Relation to Baraminology Roger W. Sanders Bryan College Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Sanders, Roger W. (2013) "The Fossil Record of Angiosperm Families in Relation to Baraminology," The Proceedings of the International Conference on Creationism: Vol. 7 , Article 31. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol7/iss1/31 Proceedings of the Seventh International Conference on Creationism. Pittsburgh, PA: Creation Science Fellowship THE FOSSIL RECORD OF ANGIOSPERM FAMILIES IN RELATION TO BARAMINOLOGY Roger W. Sanders, Ph.D., Bryan College #7802, 721 Bryan Drive, Dayton, TN 37321 USA KEYWORDS: Angiosperms, flowering plants, fossils, baramins, Flood, post-Flood continuity criterion, continuous fossil record ABSTRACT To help estimate the number and boundaries of created kinds (i.e., baramins) of flowering plants, the fossil record has been analyzed. To designate the status of baramin, a criterion is applied that tests whether some but not all of a group’s hierarchically immediate subgroups have a fossil record back to the Flood (accepted here as near the Cretaceous-Paleogene boundary). -

By C. D. K. COOK the Sparganiaceae Were Monographed by Graebner
SPARGANIUM IN BRITAIN By C. D. K. COOK Institut fur systematische Botanik der Universitat Munchen The Sparganiaceae were monographed by Graebner (1900) in Das Pf/anzenreich. About seven years later Professor Wladislaw Rothert, formerly at the University of Odes sa, started to prepare a more comprehensive monograph. Unfortunately Rothert suffered the tragic fate of so lllany elderly Polish and Russian scientists in the Russian Revolution and his work seems to have been lost. In 1911, however, he visited the herbaria at Kew and the British Museum and wrote notes on many of the herbarium sheets. I have used these notes as a guide to the taxonomy. SPARGANIUM L., Sp. PI., 971 (1753). Glabrous aquatic (occasionally semi-terrestrial) perennial herbs, reproducing vegeta tively by long, thin, underground rhizomes. 'Stems simple or branched. Leaves linear, distichous, sheathing at the base, erect or floating. Flowers unisexual, crowded into separate globose capitula, the female capi.tula towards the base in each inflorescence; perianth of 3 - 6 radiate scales; male flowers of 3 - 8 stamens, the filaments sometimes partially united; female flowers of one, occasionally two, rarely three, fused carpels with a single style persisting in fruit, and as many stigmas as carpels. Fruit drupaceous with a dry, spongy exocarp, and a hard endocarp; seed albuminous, with a large embryo. Pollination mainly by wind. There are about fifteen species in the North Temperate regions, extending from sub arctic Scandinavia and North America to the Mediterranean and Mississippi Basin; the distributional belt stretches across North America, Europe, and Asia to Japan. Two species . occur in South Australia and New Zealand. -

Phylogenetic Relationships of Monocots Based on the Highly Informative Plastid Gene Ndhf Thomas J
Aliso: A Journal of Systematic and Evolutionary Botany Volume 22 | Issue 1 Article 4 2006 Phylogenetic Relationships of Monocots Based on the Highly Informative Plastid Gene ndhF Thomas J. Givnish University of Wisconsin-Madison J. Chris Pires University of Wisconsin-Madison; University of Missouri Sean W. Graham University of British Columbia Marc A. McPherson University of Alberta; Duke University Linda M. Prince Rancho Santa Ana Botanic Gardens See next page for additional authors Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Givnish, Thomas J.; Pires, J. Chris; Graham, Sean W.; McPherson, Marc A.; Prince, Linda M.; Patterson, Thomas B.; Rai, Hardeep S.; Roalson, Eric H.; Evans, Timothy M.; Hahn, William J.; Millam, Kendra C.; Meerow, Alan W.; Molvray, Mia; Kores, Paul J.; O'Brien, Heath W.; Hall, Jocelyn C.; Kress, W. John; and Sytsma, Kenneth J. (2006) "Phylogenetic Relationships of Monocots Based on the Highly Informative Plastid Gene ndhF," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 22: Iss. 1, Article 4. Available at: http://scholarship.claremont.edu/aliso/vol22/iss1/4 Phylogenetic Relationships of Monocots Based on the Highly Informative Plastid Gene ndhF Authors Thomas J. Givnish, J. Chris Pires, Sean W. Graham, Marc A. McPherson, Linda M. Prince, Thomas B. Patterson, Hardeep S. Rai, Eric H. Roalson, Timothy M. Evans, William J. Hahn, Kendra C. Millam, Alan W. Meerow, Mia Molvray, Paul J. Kores, Heath W. O'Brien, Jocelyn C. Hall, W. John Kress, and Kenneth J. Sytsma This article is available in Aliso: A Journal of Systematic and Evolutionary Botany: http://scholarship.claremont.edu/aliso/vol22/iss1/ 4 Aliso 22, pp. -

Diversityand Classification of Flowering Plants
180 CHAPTER 6 EVOLUTION OF FLOWERING PLANTS F REFERENCES FOR FURTHER STUDY Andrews, H. N. 1961. Studies in Paleobotany. Wiley, New York. APG ifi. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105—121. Crane, P. R., E. M. Friis, and K. Pedersen. 1995. The origin and early diversification of angiosperms. Nature 374: 27. 7 Crepet, W. L. 1998. The abominable mystery. Science 282: 1653—1654. Cronquist, A. 1981. An integrated system of classification of flowering plants. Columbia University Press, New York. Davies, T. J., T. G. Barraclough, M. W. Chase, P. 5. Soltis, D. E. Soltis, and V. Savolainen. 2004. Darwin’s abominable mystery: Insights from AND CLASSIFICATION a supertree of DIVERSITY the angiosperms. Proceedings of the National Academy of Sciences of the United States of America 101: 1904—1909. Doyle, J. A. 2006. Seed ferns and the origin of angiosperms. Journal of the Torrey Botanical Society 133: 169—209. Doyle, J. A. 2008. Integrating molecular phylogenetic and paleobotanical evidence on origin of the flower. International Journal of Plant FLOWERING PLANTS: Sciences 169: 816—843. OF Eames, A. J. 1961. Morphology of the angiosperms. McGraw Hill, New York. Friedman, W. and E., J. H. Williams. 2004. Developmental evolution of the sexual process in ancient flowering plant lineages. The Plant Cell NYMPHAEALE S, 16, S119—S 132, Supplement. AMBORELLALE S, Friedman, W. E., and K. C. Ryerson. 2009. Reconstructing the ancestral female gametophyte of angiosperms: insights from Amborella and other ancient lineages of flowering plants. -

2 ANGIOSPERM PHYLOGENY GROUP (APG) SYSTEM History Of
ANGIOSPERM PHYLOGENY GROUP (APG) SYSTEM The Angiosperm Phylogeny Group, or APG, refers to an informal international group of systematic botanists who came together to try to establish a consensus view of the taxonomy of flowering plants (angiosperms) that would reflect new knowledge about their relationships based upon phylogenetic studies. As of 2010, three incremental versions of a classification system have resulted from this collaboration (published in 1998, 2003 and 2009). An important motivation for the group was what they viewed as deficiencies in prior angiosperm classifications, which were not based on monophyletic groups (i.e. groups consisting of all the descendants of a common ancestor). APG publications are increasingly influential, with a number of major herbaria changing the arrangement of their collections to match the latest APG system. Angiosperm classification and the APG Until detailed genetic evidence became available, the classification of flowering plants (also known as angiosperms, Angiospermae, Anthophyta or Magnoliophyta) was based on their morphology (particularly that of the flower) and their biochemistry (what kinds of chemical compound they contained or produced). Classification systems were typically produced by an individual botanist or by a small group. The result was a large number of such systems (see List of systems of plant taxonomy). Different systems and their updates tended to be favoured in different countries; e.g. the Engler system in continental Europe; the Bentham & Hooker system in Britain (particularly influential because it was used by Kew); the Takhtajan system in the former Soviet Union and countries within its sphere of influence; and the Cronquist system in the United States. -

Intraspecific Differentiation of Sparganium Erectum in the Czech Republic: Molecular, Genome Size and Morphometric Analysis
Preslia 92: 137–165, 2020 137 Intraspecific differentiation of Sparganium erectum in the Czech Republic: molecular, genome size and morphometric analysis Vnitrodruhová diferenciace Sparganium erectum v České republice: molekulární a morfometrické analýzy a stanovení velikosti genomu Soňa Píšová1,2 & Tomáš Fér1 1Department of Botany, Faculty of Science, Charles University, Benátská 2, CZ-128 00 Prague, Czech Republic, e-mail: [email protected]; 2Czech Academy of Sciences, Institute of Botany, CZ-252 43 Průhonice, Czech Republic, e-mail: [email protected] Píšová S. & Fér T. (2020) Intraspecific differentiation of Sparganium erectum in the Czech Republic: molecular, genome size and morphometric analysis. – Preslia 92: 137–165. Aquatic and wetland plants tend to be very phenotypically plastic, which accounts for the taxo- nomic difficulties in many groups. In the genus Sparganium, which comprises about 14 species, numerous taxa at different ranks are described. The classification of the genus is based on genera- tive characters on the fruit, which are less influenced by the environment than vegetative charac- ters. Nevertheless, the intraspecific division of Sparganium erectum poses problems, especially the existence of several intraspecific taxa along with intermediate individuals. In this study we examined four European subspecies of S. erectum (subsp. erectum, subsp. microcarpum, subsp. neglectum and subsp. oocarpum) from 64 populations in the Czech Republic. A combination of multivariate morphometrics, AFLPs and genome size estimation allowed us to confirm the cur- rent subspecies classification and investigate putative intraspecific hybridization. Four genetic groups with different genome sizes corresponding to the subspecies were found. Morphological characters that were described in previous studies correlated with these genetic groups and thus affirmed the classification. -
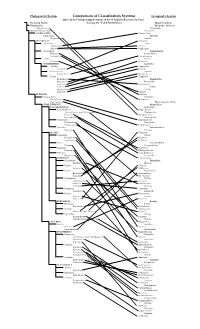
Comparison of Cronquist and Phylogeneticalt
Phylogenetic System Comparison of Classification Systems Cronquist's System Lines Show Corresponding Positions of the 60 Families Reviewed in Class Flowring Plants Biology 211 - Fall 2002 (Phillips) Magnoliophyta Nymphaeales Liliopsida - Monocots Nymphaeaceae Alismatidae (unnamed major clade) Alismatales MAGNOLIIDS Alismataceae Magnoliales Arecidae Magnoliaceae Arecales MONOCOTS Arecaceae Alismatales Arales Alismataceae Araceae Araceae (incl. Lemnaceae) Lemnaceae Asparagales Commelinidae Iridaceae Commelinales Orchidaceae Commelinaceae Liliales Juncales Liliaceae Juncaceae COMMELINIDS Cyperales Arecales Cyperaceae Arecaceae Poaceae Commelinales Typhales Commelinaceae Sparganiaceae Poales Typhaceae Bromeliaceae Zingiberidae Cyperaceae Bromeliales Juncaceae Bromeliaceae Poaceae Lilidae Sparganiaceae Liliales Typhaceae Liliaceae EUDICOTS Iridaceae Ranunculales Orchidales Ranunculaceae Orchidaceae Papaveraceae Magnoliopsida - Dicots CORE EUDICOTS Magnoliidae Caryophyllid Clade Magnoliales Caryophyllales Magnoliaceae Amaranthaceae (incl. Chenopodiaceae) Nymphaeales Cactaceae Nymphaeaceae Caryophyllaceae Ranunculales Polygonales Ranunculaceae Droseraceae Papaverales Polygonaceae Papaveraceae Santalales Hamamelididae Viscaceae Urticales ROSIDS Ulmaceae Saxifragales Fagales Crassulaceae Fagaceae Saxifragaceae Betulaceae Vitales Caryophyllidae Vitaceae Caryophyllales Geraniales Cactaceae Geraniaceae Chenopodiaceae Myrtales Caryophyllaceae Onagraceae Polygonales EUROSIDS I Polygonaceae Cucurbitales Dilleniidae Cucurbitaceae Malvales Fabales Tiliaceae -

Chromosome Studies in Indian Pandanales
402 Cytologia 31 Chromosome Studies in Indian Pandanales Ranajit Mallick and Arun Kumar Sharma Cytogenetics Laboratory, Department of Botany, University of Calcutta, 35, Ballygunj Circular Road, Calcutta 19, India Received November 2, 1965 Introduction The systematic positions of the three monocotyledonous genera Pandanus, Typha and Sparganium have been much debated. Though the three have been placed in different families by all taxonomists, the principal controversy involves their affinities. Engler and Prantl (1930) included all the three families under one order Pandanales in their system of classification. Hutchinson (1959), on the other hand, separated Typhaceae and Sparganiaceae under the order Typhales and Pandanaceae under Pandanales. Moreover in the latter system the two orders are widely separated from one another. In addition to the positions of the three genera the status of Pandanales in evolution is also a matter of controversy. Radically different views are held by Engler and Prantl on one hand and Hutchinson on the other. The simplicity of the flower, that is unisexual and naked with anemophilous method of pollination, led Engler and Prantl to consider Pandanales as the most primitive order of the monocotyledons. As such it has been placed at the beginning of the series. These authors consider that evolution is progressive and increase in complexity in structure is the principal feature in evolution. Hutchinson considered regression as one of the principal features in evolution and regarded that decrease in complexity should not be ignored in the study of evolution. According to him the simplicity of Pandanales is not due to primitiveness but due to re duction and extreme specialisation of apparently simple forms. -

Angiosperm Phylogeny Group (APG) System
Angiosperm Phylogeny Group (APG) system The Angiosperm Phylogeny Group, or APG, refers to an informal international group of systematic botanists who came together to try to establish a consensus view of the taxonomy of flowering plants (angiosperms) that would reflect new knowledge about their relationships based upon phylogenetic studies. As of 2010, three incremental versions of a classification system have resulted from this collaboration (published in 1998, 2003 and 2009). An important motivation for the group was what they viewed as deficiencies in prior angiosperm classifications, which were not based on monophyletic groups (i.e. groups consisting of all the descendants of a common ancestor). APG publications are increasingly influential, with a number of major herbaria changing the arrangement of their collections to match the latest APG system. Angiosperm classification and the APG Until detailed genetic evidence became available, the classification of flowering plants (also known as angiosperms, Angiospermae , Anthophyta or Magnoliophyta ) was based on their morphology (particularly that of the flower) and their biochemistry (what kinds of chemical compound they contained or produced). Classification systems were typically produced by an individual botanist or by a small group. The result was a large number of such systems (see List of systems of plant taxonomy). Different systems and their updates tended to be favoured in different countries; e.g. the Engler system in continental Europe; the Bentham & Hooker system in Britain (particularly influential because it was used by Kew); the Takhtajan system in the former Soviet Union and countries within its sphere of influence; and the Cronquist system in the United States. -

Common Plants of the Upper Klamath Basin
Common Plants of the Upper Klamath Basin Technical Layout & Design .........Michael Calonje Editor ................................................Sarah Malaby Plant Descriptions & Text ..............Molly Juillerat, Ron Larson, Sarah Malaby, Jeanne Skalka. Photography ...........Michael Calonje, Ron Larson, Sarah Malaby, Terry Spivey. A Special acknowledgement to Klamath County Commissioners Al Switzer, John Elliott and Bill Brown for providing funding for publication costs through PL 06-393 Title III “Secure Rural Schools and Community Self-Determination Act of 2000” Oregon Native Plant Society - Klamath Basin Chapter Rabe Consulting 2007 CONTENTS Introduction ...................................................................................3 Overview ........................................................................................3 Habitats ..........................................................................................4 Plant Exploration in the Upper Klamath Basin .........................6 Growing Native Plants ..................................................................6 Species Groups ..............................................................................7 Ferns and Horsetails ..................................................................7 Conifers .....................................................................................8 Flowering Plants: Flowers, Hardwood Trees, and Shrubs .......9 Flowering Plants: Grasses and Grass-like Plants ...................9 Lichens, Bryophytes, and Blue-green -

2023 Sparganium (Typhaceae) Is an Aquatic Genus of ± 14 Species
American Journal of Botany 100(10): 2023–2039. 2013. S YSTEMATICS, BIOGEOGRAPHY, AND CHARACTER EVOLUTION OF SPARGANIUM (TYPHACEAE): DIVERSIFICATION OF A WIDESPREAD, AQUATIC LINEAGE 1 J OSHUA D. SULMAN 2,5 , B RYAN T . D REW 3 , C HLOE D RUMMOND 2 , E ISUKE H AYASAKA 4 , AND K ENNETH J. SYTSMA 2 2 Department of Botany, University of Wisconsin, Madison, Wisconsin 53706 USA; 3 Department of Biology, University of Florida, Gainesville, Florida 32607 USA; and 4 Fukui Botanical Garden, Echizen, 916-0146 Japan • Premise of the study: Sparganium (Typhaceae) is a genus of aquatic monocots containing ± 14 species, with fl owers aggregated in unisexual, spherical heads, and habit ranging from fl oating to emergent. Sparganium presents an opportunity to investigate diversifi cation, character evolution, and biogeographical relationships in a widespread temperate genus of aquatic monocots. We present a fossil-calibrated, molecular phylogeny of Sparganium based on analysis of two chloroplast and two nuclear mark- ers. Within this framework, we examine character evolution in both habit and stigma number and infer the ancestral area and biogeographic history of the genus. • Methods: Sequence data from two cpDNA and two nDNA markers were analyzed using maximum parsimony, maximum likelihood, and Bayesian inference. We used the program BEAST to simultaneously estimate phylogeny and divergence times, S-DIVA and Lagrange for biogeographical reconstruction, and BayesTraits to examine locule number and habit evolution. • Key results: Two major clades were recovered with strong support: one composed of S. erectum and S. eurycarpum ; and the other containing all remaining Sparganium . We realigned the subgenera to conform to these clades.