The Principles of Thermodynamics by N.D. Hari Dass
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Thermodynamics of Ideal Gases
D Thermodynamics of ideal gases An ideal gas is a nice “laboratory” for understanding the thermodynamics of a fluid with a non-trivial equation of state. In this section we shall recapitulate the conventional thermodynamics of an ideal gas with constant heat capacity. For more extensive treatments, see for example [67, 66]. D.1 Internal energy In section 4.1 we analyzed Bernoulli’s model of a gas consisting of essentially 1 2 non-interacting point-like molecules, and found the pressure p = 3 ½ v where v is the root-mean-square average molecular speed. Using the ideal gas law (4-26) the total molecular kinetic energy contained in an amount M = ½V of the gas becomes, 1 3 3 Mv2 = pV = nRT ; (D-1) 2 2 2 where n = M=Mmol is the number of moles in the gas. The derivation in section 4.1 shows that the factor 3 stems from the three independent translational degrees of freedom available to point-like molecules. The above formula thus expresses 1 that in a mole of a gas there is an internal kinetic energy 2 RT associated with each translational degree of freedom of the point-like molecules. Whereas monatomic gases like argon have spherical molecules and thus only the three translational degrees of freedom, diatomic gases like nitrogen and oxy- Copyright °c 1998{2004, Benny Lautrup Revision 7.7, January 22, 2004 662 D. THERMODYNAMICS OF IDEAL GASES gen have stick-like molecules with two extra rotational degrees of freedom or- thogonally to the bridge connecting the atoms, and multiatomic gases like carbon dioxide and methane have the three extra rotational degrees of freedom. -

Thermodynamics and Phase Diagrams
Thermodynamics and Phase Diagrams Arthur D. Pelton Centre de Recherche en Calcul Thermodynamique (CRCT) École Polytechnique de Montréal C.P. 6079, succursale Centre-ville Montréal QC Canada H3C 3A7 Tél : 1-514-340-4711 (ext. 4531) Fax : 1-514-340-5840 E-mail : [email protected] (revised Aug. 10, 2011) 2 1 Introduction An understanding of phase diagrams is fundamental and essential to the study of materials science, and an understanding of thermodynamics is fundamental to an understanding of phase diagrams. A knowledge of the equilibrium state of a system under a given set of conditions is the starting point in the description of many phenomena and processes. A phase diagram is a graphical representation of the values of the thermodynamic variables when equilibrium is established among the phases of a system. Materials scientists are most familiar with phase diagrams which involve temperature, T, and composition as variables. Examples are T-composition phase diagrams for binary systems such as Fig. 1 for the Fe-Mo system, isothermal phase diagram sections of ternary systems such as Fig. 2 for the Zn-Mg-Al system, and isoplethal (constant composition) sections of ternary and higher-order systems such as Fig. 3a and Fig. 4. However, many useful phase diagrams can be drawn which involve variables other than T and composition. The diagram in Fig. 5 shows the phases present at equilibrium o in the Fe-Ni-O2 system at 1200 C as the equilibrium oxygen partial pressure (i.e. chemical potential) is varied. The x-axis of this diagram is the overall molar metal ratio in the system. -

Comparative Examination of Nonequilibrium Thermodynamic Models of Thermodiffusion in Liquids †
Proceedings Comparative Examination of Nonequilibrium Thermodynamic Models of Thermodiffusion in Liquids † Semen N. Semenov 1,* and Martin E. Schimpf 2 1 Institute of Biochemical Physics RAS, Moscow 119334, Russia 2 Department of Chemistry, Boise State University, Boise, ID 83725, USA; [email protected] * Correspondence: [email protected] † Presented at the 5th International Electronic Conference on Entropy and Its Applications, 18–30 November 2019; Available online: https://ecea-5.sciforum.net/. Published: 17 November 2019 Abstract: We analyze existing models for material transport in non-isothermal non-electrolyte liquid mixtures that utilize non-equilibrium thermodynamics. Many different sets of equations for material have been derived that, while based on the same fundamental expression of entropy production, utilize different terms of the temperature- and concentration-induced gradients in the chemical potential to express the material flux. We reason that only by establishing a system of transport equations that satisfies the following three requirements can we obtain a valid thermodynamic model of thermodiffusion based on entropy production, and understand the underlying physical mechanism: (1) Maintenance of mechanical equilibrium in a closed steady-state system, expressed by a form of the Gibbs–Duhem equation that accounts for all the relevant gradients in concentration, temperature, and pressure and respective thermodynamic forces; (2) thermodiffusion (thermophoresis) is zero in pure unbounded liquids (i.e., in the absence of wall effects); (3) invariance in the derived concentrations of components in a mixture, regardless of which concentration or material flux is considered to be the dependent versus independent variable in an overdetermined system of material transport equations. The analysis shows that thermodiffusion in liquids is based on the entropic mechanism. -

Thermodynamic Temperature
Thermodynamic temperature Thermodynamic temperature is the absolute measure 1 Overview of temperature and is one of the principal parameters of thermodynamics. Temperature is a measure of the random submicroscopic Thermodynamic temperature is defined by the third law motions and vibrations of the particle constituents of of thermodynamics in which the theoretically lowest tem- matter. These motions comprise the internal energy of perature is the null or zero point. At this point, absolute a substance. More specifically, the thermodynamic tem- zero, the particle constituents of matter have minimal perature of any bulk quantity of matter is the measure motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., non- mechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational motions” are almost always in the classical dynamic temperature is often also called absolute tem- regime. Translational motions are ordinary, whole-body perature, for two reasons: one, proposed by Kelvin, that movements in three-dimensional space in which particles it does not depend on the properties of a particular mate- move about and exchange energy in collisions. Figure 1 rial; two that it refers to an absolute zero according to the below shows translational motion in gases; Figure 4 be- properties of the ideal gas. low shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature The International System of Units specifies a particular at which the particle constituents of matter are as close as scale for thermodynamic temperature. -

Equation of State - Wikipedia, the Free Encyclopedia 頁 1 / 8
Equation of state - Wikipedia, the free encyclopedia 頁 1 / 8 Equation of state From Wikipedia, the free encyclopedia In physics and thermodynamics, an equation of state is a relation between state variables.[1] More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions associated with the matter, such as its temperature, pressure, volume, or internal energy. Equations of state are useful in describing the properties of fluids, mixtures of fluids, solids, and even the interior of stars. Thermodynamics Contents ■ 1 Overview ■ 2Historical ■ 2.1 Boyle's law (1662) ■ 2.2 Charles's law or Law of Charles and Gay-Lussac (1787) Branches ■ 2.3 Dalton's law of partial pressures (1801) ■ 2.4 The ideal gas law (1834) Classical · Statistical · Chemical ■ 2.5 Van der Waals equation of state (1873) Equilibrium / Non-equilibrium ■ 3 Major equations of state Thermofluids ■ 3.1 Classical ideal gas law Laws ■ 4 Cubic equations of state Zeroth · First · Second · Third ■ 4.1 Van der Waals equation of state ■ 4.2 Redlich–Kwong equation of state Systems ■ 4.3 Soave modification of Redlich-Kwong State: ■ 4.4 Peng–Robinson equation of state Equation of state ■ 4.5 Peng-Robinson-Stryjek-Vera equations of state Ideal gas · Real gas ■ 4.5.1 PRSV1 Phase of matter · Equilibrium ■ 4.5.2 PRSV2 Control volume · Instruments ■ 4.6 Elliott, Suresh, Donohue equation of state Processes: -

Chem 322, Physical Chemistry II: Lecture Notes
Chem 322, Physical Chemistry II: Lecture notes M. Kuno December 20, 2012 2 Contents 1 Preface 1 2 Revision history 3 3 Introduction 5 4 Relevant Reading in McQuarrie 9 5 Summary of common equations 11 6 Summary of common symbols 13 7 Units 15 8 Math interlude 21 9 Thermo definitions 35 10 Equation of state: Ideal gases 37 11 Equation of state: Real gases 47 12 Equation of state: Condensed phases 55 13 First law of thermodynamics 59 14 Heat capacities, internal energy and enthalpy 79 15 Thermochemistry 111 16 Entropy and the 2nd and 3rd Laws of Thermodynamics 123 i ii CONTENTS 17 Free energy (Helmholtz and Gibbs) 159 18 The fundamental equations of thermodynamics 179 19 Application of Free Energy to Phase Transitions 187 20 Mixtures 197 21 Equilibrium 223 22 Recap 257 23 Kinetics 263 Chapter 1 Preface What is the first law of thermodynamics? You do not talk about thermodynamics What is the second law of thermodynamics? You do not talk about thermodynamics 1 2 CHAPTER 1. PREFACE Chapter 2 Revision history 2/05: Original build 11/07: Corrected all typos caught to date and added some more material 1/08: Added more stuff and demos. Made the equilibrium section more pedagogical. 5/08: Caught more typos and made appropriate fixes to some nomenclature mistakes. 12/11: Started the next round of text changes and error corrections since I’m teaching this class again in spring 2012. Woah! I caught a whole bunch of problems that were present in the last version. It makes you wonder what I was thinking. -

Physical Chemistry I
Physical Chemistry I Andrew Rosen August 19, 2013 Contents 1 Thermodynamics 5 1.1 Thermodynamic Systems and Properties . .5 1.1.1 Systems vs. Surroundings . .5 1.1.2 Types of Walls . .5 1.1.3 Equilibrium . .5 1.1.4 Thermodynamic Properties . .5 1.2 Temperature . .6 1.3 The Mole . .6 1.4 Ideal Gases . .6 1.4.1 Boyle’s and Charles’ Laws . .6 1.4.2 Ideal Gas Equation . .7 1.5 Equations of State . .7 2 The First Law of Thermodynamics 7 2.1 Classical Mechanics . .7 2.2 P-V Work . .8 2.3 Heat and The First Law of Thermodynamics . .8 2.4 Enthalpy and Heat Capacity . .9 2.5 The Joule and Joule-Thomson Experiments . .9 2.6 The Perfect Gas . 10 2.7 How to Find Pressure-Volume Work . 10 2.7.1 Non-Ideal Gas (Van der Waals Gas) . 10 2.7.2 Ideal Gas . 10 2.7.3 Reversible Adiabatic Process in a Perfect Gas . 11 2.8 Summary of Calculating First Law Quantities . 11 2.8.1 Constant Pressure (Isobaric) Heating . 11 2.8.2 Constant Volume (Isochoric) Heating . 11 2.8.3 Reversible Isothermal Process in a Perfect Gas . 12 2.8.4 Reversible Adiabatic Process in a Perfect Gas . 12 2.8.5 Adiabatic Expansion of a Perfect Gas into a Vacuum . 12 2.8.6 Reversible Phase Change at Constant T and P ...................... 12 2.9 Molecular Modes of Energy Storage . 12 2.9.1 Degrees of Freedom . 12 1 2.9.2 Classical Mechanics . -

Definition of the Ideal Gas
ON THE DEFINITION OF THE IDEAL GAS. By Edgar Bucidngham. I . Nature and purpose of the definition.—The notion of the ideal gas is that of a gas having particularly simple physical properties to which the properties of the real gases may be considered as approximations; or of a standard to which the real gases may be referred, the properties of the ideal gas being simply defined and the properties of the real gases being then expressible as the prop- erties of the standard plus certain corrections which pertain to the individual gases. The smaller these corrections the more nearly the real gas approaches to being in the "ideal state." This con- ception grew naturally from the fact that the earlier experiments on gases showed that they did not differ much in their physical properties, so that it was possible to define an ideal standard in such a way that the corrections above referred to should in fact all be ''small" in terms of the unavoidable errors of experiment. Such a conception would hardly arise to-day, or if it did, would not be so simple as that which has come down to us from earlier times when the art of experimenting upon gases was less advanced. It is evident that a quantitative definition of the ideal standard gas needs to be more or less complete and precise according to the nature of the problem under immediate consideration. If changes of temperature and of internal energy play no part, all that is usually needed is a standard relation between pressure and volume. -

Thermodynamics
...Thermodynamics Clausius Inequality R dq Existence of a definite integral T Entropy - a state variable Second Law stated as principle of increasing entropy Entropy changes in system and the surroundings October 9, 2018 1 / 20 The Principle of Increasing Entropy Isolated system, or System plus Surroundings enclosed by an adiabatic wall, or entire universe. d¯q = 0 ) ∆S ≥ 0. The entropy of a thermally isolated ensemble can never decrease. This defines a direction of time. The Second Law is the ONLY fundamental equation in physics which violates CPT Symmetry. Boltzmann argued that its validity was not based on the inherent laws of nature but rather on the choice of extremely improbable initial conditions. October 9, 2018 2 / 20 The Central Equation of Thermodynamics Combine the first and second laws. dU = d¯Q + d¯W ! dU = TdS - PdV Derived for infinitesimal reversible processes in differential form. This equation involves only state variables. Can be integrated to relate changes of state variables independent of the path of integration. Integration of equation can be applied to irreversible processes. Can be written TdS = dU + PdV , relating entropy to more easily measurable quantities. U and V are extensive quantities, so S must also be extensive. October 9, 2018 3 / 20 Caveat: PdV implies mechanical work compressing a fluid. In general d¯W should include all forms of work. e.g. For electric charge (Z) and emf E, dU = TdS − PdV + EdZ. For a rubber band length L under tension F, dU = TdS + FdL. October 9, 2018 4 / 20 Entropy of an ideal gas For an Ideal gas U = U(T ), so dU = Cv dT . -

Evaluation of Thermodynamic Equations of State Across Chemistry and Structure in the Materials Project
Lawrence Berkeley National Laboratory Recent Work Title Evaluation of thermodynamic equations of state across chemistry and structure in the materials project Permalink https://escholarship.org/uc/item/0v70b5jx Journal npj Computational Materials, 4(1) ISSN 2057-3960 Authors Latimer, K Dwaraknath, S Mathew, K et al. Publication Date 2018-12-01 DOI 10.1038/s41524-018-0091-x Peer reviewed eScholarship.org Powered by the California Digital Library University of California www.nature.com/npjcompumats ARTICLE OPEN Evaluation of thermodynamic equations of state across chemistry and structure in the materials project Katherine Latimer1, Shyam Dwaraknath2, Kiran Mathew3, Donald Winston2 and Kristin A. Persson2,3 Thermodynamic equations of state (EOS) for crystalline solids describe material behaviors under changes in pressure, volume, entropy and temperature, making them fundamental to scientific research in a wide range of fields including geophysics, energy storage and development of novel materials. Despite over a century of theoretical development and experimental testing of energy–volume (E–V) EOS for solids, there is still a lack of consensus with regard to which equation is indeed optimal, as well as to what metric is most appropriate for making this judgment. In this study, several metrics were used to evaluate quality of fit for 8 different EOS across 87 elements and over 100 compounds which appear in the literature. Our findings do not indicate a clear “best” EOS, but we identify three which consistently perform well relative to the rest of the set. Furthermore, we find that for the aggregate data set, the RMSrD is not strongly correlated with the nature of the compound, e.g., whether it is a metal, insulator, or semiconductor, nor the bulk modulus for any of the EOS, indicating that a single equation can be used across a broad range of classes of materials. -

A Nonequilibrium Thermodynamics Perspective on Nature-Inspired Chemical Engineering Processes Vincent Gerbaud, Nataliya Shcherbakova, Sergio Da Cunha
A nonequilibrium thermodynamics perspective on nature-inspired chemical engineering processes Vincent Gerbaud, Nataliya Shcherbakova, Sergio da Cunha To cite this version: Vincent Gerbaud, Nataliya Shcherbakova, Sergio da Cunha. A nonequilibrium thermodynamics per- spective on nature-inspired chemical engineering processes. Chemical Engineering Research and De- sign, Elsevier, 2019, 154, pp.316-330. 10.1016/j.cherd.2019.10.037. hal-02648825 HAL Id: hal-02648825 https://hal.archives-ouvertes.fr/hal-02648825 Submitted on 29 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/ 26045 Official URL : https://doi.org/10.1016/j.cherd.2019.10.037 To cite this version: Gerbaud, Vincent and Da Cunha, Sergio and Shcherbakova, Nataliya A nonequilibrium thermodynamics perspective on nature-inspired chemical engineering -
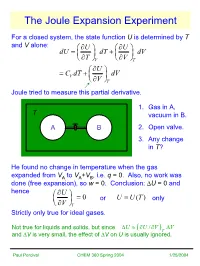
The Joule Expansion Experiment
The Joule Expansion Experiment For a closed system, the state function U is determined by T and V alone: ∂UU ∂ dU=+ dT dV ∂∂TVVT ∂U =+CdTV dV ∂V T Joule tried to measure this partial derivative. 1. Gas in A, T vacuum in B. AB2. Open valve. 3. Any change in T? He found no change in temperature when the gas expanded from VA to VA+VB, i.e. q = 0. Also, no work was done (free expansion), so w = 0. Conclusion: ∆U = 0 and hence ∂U = 0 orUUT= () only ∂V T Strictly only true for ideal gases. Not true for liquids and solids, but since ∆UUVV≈∂/ ∂ ∆ ( )T and ∆V is very small, the effect of ∆V on U is usually ignored. Paul Percival CHEM 360 Spring 2004 1/25/2004 Pressure Dependence of Enthalpy For a closed system, the state function H is determined by T and P alone: ∂HH ∂ dH=+ dT dP ∂∂TPPT ∂H =+CdTP dP ∂P T For ideal gases HUPVUnRT=+ =+ ∂∂HU = = 0 ∂∂PPTT Not true for real gases, liquids and solids! dH=+ dU PdV + VdP ∂∂HU CPV dT+=+++ dP C dT dV PdV VdP ∂∂PVTT At fixed temperature, dT = 0 ∂∂∂∂HUVV =++PV ≈ V ∂∂∂∂PVPPTTTT small for liquids and solids Investigate real gases at constant enthalpy, i.e. dH = 0 ∂∂HT ⇒ =−CP ∂∂PPTH Paul Percival CHEM 360 Spring 2004 1/25/2004 The Joule-Thomson Experiment ∂T Joule-Thomson Coefficient µ=JT ∂P H Pump gas through throttle (hole or porous plug) P1 > P2 P1 Keep pressures constant by moving pistons. Work done by system −=wPVPV22 − 11 Since q = 0 P2 ∆=UU21 − U == wPVPV 1122 − ⇒ UPVUPV222111+=+ HH21= constant enthalpy Measure change in T of gas as it moves from side 1 to side 2.