The Crystal Structure of Stannite, Cu2fesns4. by L
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Stannoidite Cu8(Fe, Zn)3Sn2s12 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic
Stannoidite Cu8(Fe, Zn)3Sn2S12 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 222. Massive, in veinlets, to 1 mm. Physical Properties: Fracture: Uneven to subconchoidal. Hardness = ∼4 VHN = 181–274 (100 g load). D(meas.) = n.d. D(calc.) = [4.68] Optical Properties: Opaque. Color: Brass-brown; in polished section, pinkish brown. Streak: Dark brown-gray. Luster: Metallic. Pleochroism: Distinct, pale salmon-brown to brown. Anisotropism: Strong; dark orange-red to yellow-gray. R1–R2: (400) 17.5–18.9, (420) 17.4–19.2, (440) 18.2–20.3, (460) 19.5–21.7, (480) 20.8–23.0, (500) 21.8–24.2, (520) 22.8–25.3, (540) 23.7–26.3, (560) 24.6–27.1, (580) 25.4–27.8, (600) 26.2–28.4, (620) 27.1–29.1, (640) 27.8–29.5, (660) 28.7–30.0, (680) 29.6–30.5, (700) 30.3–30.8 Cell Data: Space Group: I222. a = 10.767 b = 5.411 c = 16.118 Z = [2] X-ray Powder Pattern: Konjo mine, Japan. 3.11 (100), 1.906 (70), 1.621 (20b), 2.70 (16), 4.83 (10), 5.40 (5), 4.13 (4) Chemistry: (1) (2) (1) (2) Cu 37.2 38.2 Zn 1.2 0.8 Ag 0.1 Sn 16.5 18.7 Fe 12.5 11.9 S 31.2 29.9 Total 98.7 99.5 (1) Konjo mine, Japan; by electron microprobe, corresponding to (Cu7.22Ag0.01)Σ=7.23 (Fe2.76Zn0.23)Σ=2.99Sn1.71S12.00. -

The University of Manitoba
THE OCCURRH\ICE OF TTN AT THE DtcKsroNE No. 2 oRBODY, NORTHmN IfANTTOBA by Marti:: Gray }4crcice Trliruli-peg, Manitoba Iftarcln, l97l+ A Thesis Submitted to the Faculty of Graduate SbudÍes and Research The University of Manitoba I:i Part,ial Fït]-fillrnent of the Requirements for the Degree of Master of Science ABSIRACT The Dickstone No. 2 Orebody, a sna1l massive sulphide deposít situated i¡r northern Ïvlanitoba, contain" lirl i¡ amou::ts averaging O"O8/" or 1o6 pounds per ton. ' Most of the tin occurs-as the oxide cassiterite. The cassiterite shows a considerable size range from (.OOI fiÍn. to 0o6 tmno, with approxÍ:nately 99% of a-ll cassiterite present Ín graÍns larger than Oo1 nm. acrossc_ The larger cassiterite grains e:dribit subhedral crystal forms, Some of which show the e'ffects of abrasion. Quafr'zt calcite and ' pyrite are most closely associated with the cassiterite u A microprobe study of the'cassiterites shows that the most connnon trace elem.e=nts are jndium, siJver, neod¡rmium, tur:gsten, iodine and lutetium. Ca1cíum, anti-rnony, and ytterbium occur moderately, whi-le titan-iran, iron, zi:rrc, tantalurn and iridium occur infrequently. Another tin-bearing rnineral was encour:teredo .Llthough not posÍtively identified, this mjneral ís a calcium-titanium silicate, possibly sphene" l-l_ ACKNOTìILEDGEMH\TS The uriter would like to thank Dro H. Du Bo lniilson for his help and supervision durirrg the preparation of this thesi-s. Aclcrow- ledgements are al-so exbended to Mro Ku RamJ-al- whose guidance dwi:rg the electron rnícroprobe phase of this study proved invaluable. -

Tungsten Minerals and Deposits
DEPARTMENT OF THE INTERIOR FRANKLIN K. LANE, Secretary UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, Director Bulletin 652 4"^ TUNGSTEN MINERALS AND DEPOSITS BY FRANK L. HESS WASHINGTON GOVERNMENT PRINTING OFFICE 1917 ADDITIONAL COPIES OF THIS PUBLICATION MAY BE PROCURED FROM THE SUPERINTENDENT OF DOCUMENTS GOVERNMENT PRINTING OFFICE WASHINGTON, D. C. AT 25 CENTS PER COPY CONTENTS. Page. Introduction.............................................................. , 7 Inquiries concerning tungsten......................................... 7 Survey publications on tungsten........................................ 7 Scope of this report.................................................... 9 Technical terms...................................................... 9 Tungsten................................................................. H Characteristics and properties........................................... n Uses................................................................. 15 Forms in which tungsten is found...................................... 18 Tungsten minerals........................................................ 19 Chemical and physical features......................................... 19 The wolframites...................................................... 21 Composition...................................................... 21 Ferberite......................................................... 22 Physical features.............................................. 22 Minerals of similar appearance................................. -

VEIN and GREISEN SN and W DEPOSITS (MODELS 15A-C; Cox and Bagby, 1986; Reed, 1986A,B) by James E. Elliott, Robert J. Kamilli, Wi
VEIN AND GREISEN SN AND W DEPOSITS (MODELS 15a-c; Cox and Bagby, 1986; Reed, 1986a,b) by James E. Elliott, Robert J. Kamilli, William R. Miller, and K. Eric Livo SUMMARY OF RELEVANT GEOLOGIC, GEOENVIRONMENTAL, AND GEOPHYSICAL INFORMATION Deposit geology Vein deposits consist of simple to complex fissure filling or replacement quartz veins, including discrete single veins, swarms or systems of veins, or vein stockworks, that contain mainly wolframite series minerals (huebnerite-ferberite) and (or) cassiterite as ore minerals (fig. 1). Other common minerals are scheelite, molybdenite, bismuthinite, base- etal sulfide minerals, tetrahedrite, pyrite, arsenopyrite, stannite, native bismuth, bismuthinite, fluorite, muscovite, biotite, feldspar, beryl, tourmaline, topaz, and chlorite (fig. 2). Complex uranium, thorium, rare earth element oxide minerals and phosphate minerals may be present in minor amounts. Greisen deposits consist of disseminated cassiterite and cassiterite-bearing veinlets, stockworks, lenses, pipes, and breccia (fig. 3) in gangue composed of quartz, mica, fluorite, and topaz. Veins and greisen deposits are found within or near highly evolved, rare-metal enriched plutonic rocks, especially near contacts with surrounding country rock; settings in or adjacent to cupolas of granitic batholiths are particularly favorable. Figure 1. Generalized longitudinal section through the Xihuashan and Piaotang tungsten deposits in the Dayu district, China. Other vein systems are indicated by heavy lines. I, upper limits of ore zones; II, lower limits of ore zones. Patterned area is granite batholith. Unpatterned area is sedimentary and metamorphic rocks (from Elliott, 1992). Figure 2. Maps and sections of tungsten vein deposits illustrating mineral and alteration zoning. A, Chicote Grande deposit, Bolivia; B, Xihuashan, China (from Cox and Bagby, 1986). -

ISOMORPHISM in the MINERALS of STANNITE-FAMIL Y Tat'yana L
New data on minerals. M.: 2003. Volume 38 65 UDK 548.32 ISOMORPHISM IN THE MINERALS OF STANNITE-FAMIL Y Tat'yana L. Evstigneeva /nstitllte 0/ Ge%gy 0/ Ore Deposits (/GEM RAS). Moscow, [email protected] Vyacheslav S. Rusakov Physics Department, Lomonosov Moscow State University. Moscow, [email protected] Yurii K. Kabalov Ge%gy Department, Lomonosov Moscow State University, Moscow, kaba/oV@geo/.mSll.Tll The crystals structures of stannite group minerals and mechanism of isomorphic substitution were studied using a complex of analytical techniques. Ten members of the kuramite-stannite series, Cu3xFe,SnS4 (O<x< 1). synthesized by -0.1 formula units, pure stannite, and kesterite were chosen for this study. The methods used included microprobe and profile analysis (Rietveld method). Mossbauer spectroscopy, scanning and transmit- ting electron microscopy, and X-ray photoelectron spectroscopy. The intermediate members of the kuramite- stannite series are structurally similar homogeneous phases of tetragonal symmetry with regularly changing unit cell parameters (c/a-2). According to the structural analysis, four compounds of the series CU3.,Fe,SnS4 with x = 0.3, 0.6, 0.8, and 1.0 have tetragonal structures, which differ from stannite by their lower symmetry (14) and distribution of atoms among tetrahedral positions. According to the Mossbauer, the compounds of this mineral group contain divalent and trivalent iron atoms. Below the limiting iron atom concentration Fe (x) - 0.5 data shows that all Fe atoms are trivalent and occupy the sulfur octahedra. At 0<x<0.5, the substitution of atoms and the change of the structural positions and atom valence is carried out according to the scheme: 2CuH(TdJ -? Cul+(TdJ + (TdJ + FeH(OhJ. -

Primary Minerals of the Jáchymov Ore District
Journal of the Czech Geological Society 48/34(2003) 19 Primary minerals of the Jáchymov ore district Primární minerály jáchymovského rudního revíru (237 figs, 160 tabs) PETR ONDRU1 FRANTIEK VESELOVSKÝ1 ANANDA GABAOVÁ1 JAN HLOUEK2 VLADIMÍR REIN3 IVAN VAVØÍN1 ROMAN SKÁLA1 JIØÍ SEJKORA4 MILAN DRÁBEK1 1 Czech Geological Survey, Klárov 3, CZ-118 21 Prague 1 2 U Roháèových kasáren 24, CZ-100 00 Prague 10 3 Institute of Rock Structure and Mechanics, V Holeovièkách 41, CZ-182 09, Prague 8 4 National Museum, Václavské námìstí 68, CZ-115 79, Prague 1 One hundred and seventeen primary mineral species are described and/or referenced. Approximately seventy primary minerals were known from the district before the present study. All known reliable data on the individual minerals from Jáchymov are presented. New and more complete X-ray powder diffraction data for argentopyrite, sternbergite, and an unusual (Co,Fe)-rammelsbergite are presented. The follow- ing chapters describe some unknown minerals, erroneously quoted minerals and imperfectly identified minerals. The present work increases the number of all identified, described and/or referenced minerals in the Jáchymov ore district to 384. Key words: primary minerals, XRD, microprobe, unit-cell parameters, Jáchymov. History of mineralogical research of the Jáchymov Chemical analyses ore district Polished sections were first studied under the micro- A systematic study of Jáchymov minerals commenced scope for the identification of minerals and definition early after World War II, during the period of 19471950. of their relations. Suitable sections were selected for This work was aimed at supporting uranium exploitation. electron microprobe (EMP) study and analyses, and in- However, due to the general political situation and the teresting domains were marked. -

Stannite from the Otoge Kaolin-Pyrophyllite Deposits, Yamagata Prefecture, NE Japan and Its Genetical Significance As Well As Ca
RESOURCE GEOLOGY, 44(6), 439•`444, 1994 Stannite from the Otoge Kaolin-Pyrophyllite Deposits, Yamagata Prefecture, NE Japan and Its Genetical Significance Makoto WATANABE*, Ken-ichi HOSHINO*, KO KO MYINT*, Kazunori MIYAZAKI* and Hirotugu NISHIDO** Abstract: Stannite was found in the Otoge kaolin-pyrophyllite deposits of ca. 4Ma(K-Ar), which are localized in rhyolitic pyroclastic rocks of middle Miocene time. Stannite occurs exclusively in pyrite-rich silicified portion within the sericite zone. Under the microscope, stannite, strongly anisotropic, shows a close association with sphalerite and pyrite. Microprobe analysis of stannite reveal that it is of Zn-rich variety with Fe/Fe+Zn atomic ratios of 0.49 to 0.71. It is noted that the atomic contents of the (Fe+Zn) site exceed 1.000 by up to 30% for stannite which contains more than 6 wt. % of Zn, suggesting a possibility of non-stoichiometry. The temperatures estimated based on the Fe and Zn partition are 300•‹to 340•‹C with Log fs2 of ca. -10 to -8, approximately corresponding to the corrected filling temperatures of fluid inclusions in quartz nearby stan- nite. the Yatani and Otoge deposits. The resultant ages Introduction are: 3.25•}0.26 to 3.6•}0.27 Ma on adularia; As well as cassiterite, stannite, Cu2(Fe, 5.16•}1.95 Ma on sericitized rock, respectively. Zn)SnS4 , is a mineral of rather ubiquitous occur- During the course of our investigation of the rence especially in skarn deposits and plutonic kaolin-pyrophyllite deposits at the Otoge mine, veins, which are genetically related to granitic we have found stannite. -

Distribution of in and Other Rare Metals in Cassiterite and Associated
Distribution of In and other rare metals in cassiterite and associated minerals in Sn ± W ore deposits of the western Variscan Belt Catherine Lerouge, Eric Gloaguen, Guillaume Wille, Laurent Bailly To cite this version: Catherine Lerouge, Eric Gloaguen, Guillaume Wille, Laurent Bailly. Distribution of In and other rare metals in cassiterite and associated minerals in Sn ± W ore deposits of the western Variscan Belt. European Journal of Mineralogy, Copernicus, 2017, 29 (4), pp.739 - 753. 10.1127/ejm/2017/0029- 2673. insu-01555328v2 HAL Id: insu-01555328 https://hal-insu.archives-ouvertes.fr/insu-01555328v2 Submitted on 19 Oct 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License Eur. J. Mineral. 2017, 29, 739–753 Special issue dedicated to Published online 26 June 2017 Zdeněk Johan Distribution of In and other rare metals in cassiterite and associated minerals in Sn ± W ore deposits of the western Variscan Belt 1,* 1,2 1,2 1 CATHERINE LEROUGE ,ERIC GLOAGUEN ,GUILLAUME WILLE and LAURENT BAILLY 1 BRGM, 3 Avenue Claude Guillemin, 45060 Orléans, France *Corresponding author, e-mail: [email protected] 2 ISTO, UMR 7327 - CNRS/Université d’Orléans, 1A rue de la Férollerie, 45071 Orléans Cedex 2, France Abstract: We present data available on rare metal and indium distributions in cassiterite and associated minerals from thirteen Sn ± W granite-related ore deposits in the western Variscan Belt (Massif Central and Armorican Massif, France; Galicia, Spain; and SW England). -
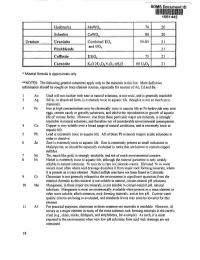
Minerals List and General Comments "More Definitive
SDMS Document ID 1061442 Huebnerite MnW04 76 20 Scheelite CaW04 80 20 Uranium Uraninite Combined U02 50-85 21 andUO Pitchblende 3 21 Coffinite USi04 75 21 Carnotite K,O2U2OgV205.nH,O 60 UA 21 * Mineral formula is approximate only. **NOTES: The following general comments apply only to the minerals in this list. More definitive information should be sought on trace element sources, especially for sources of As, Cd and Be. 1 Au Gold will not oxidize with ease in natural solutions, is not toxic, and is generally insoluble. 2 Ag Silver, in dissolved form, is extremely toxic to aquatic life, though it is not so much so to mammals. 3 Fe Iron at high concentrations may be chronically toxic to aquatic life as Fe-hydroxide may coat eggs, cement sandy or gravelly substrates, and inhibit the reproduction or growth of aquatic life of various forms. However, iron from these particular major ore minerals, is strongly insoluble in natural solutions, and therefore not of considerable environmental consequence. 4 Cu Copper is very soluble over a broad range of natural conditions, and is extremely toxic to aquatic life. 5 Pb Lead is extremely toxic to aquatic life. All of these Pb minerals require acidic solutions in order to dissolve. 6 Zn Zinc is extremely toxic to aquatic life. Zinc is commonly present as small inclusions in chalcopyrite, so should be especially evaluated in rocks that are known to contain copper • sulfides. 7 Sn Tin, much like gold, is strongly insoluble, and not of much environmental concern. 8 Ni Nickel is extremely toxic to aquatic life, although the mineral gamierite is only weakly soluble in natural solutions. -

On the Occurrence of Varlamoffte (Partially Hydrated Stannic Oxide) in Cornwall
817 On the occurrence of varlamoffte (partially hydrated stannic oxide) in Cornwall. By SIR ARTHUR RUSSELL, Bart. and E. A. VINCENT,1 B.Sc., Ph.D., A.R.I.C., F.G.S. Lecturer in Mineralogy, Durham Colleges in the University of Durham. [Read November 1, 1951.] CHARACTERS AbID MODE OF OCCURRENCE (A.R.). long ago as 1906 I had noticed a yellowish clay-like mineral occur- ~ ring somewhat abundantly at both Cligga mine, Perranzabuloe, and at Bunny mine, St. Austell. From its appearance the mineral did not look particularly interesting, neither did it suggest being one containing a metal, and for these reasons no attempt was made to deter- mine its character. In a paper read before this Society in 1937 I mentioned this yellowish clay-hke substance as occurring associated with rashleighite at the Bunny mine. 2 Soon after the publication of this paper iu 1948 I received a letter from Monsieur N. Varlamoff in the Belgian Congo saying he had seen my reference to the 'yellowish clay- like substance' (p. 356) and suggested it might possibly be identical with the mineral varlamoffite which he had recently discovered in the Belgian Congo and which had been named after him, his description of the mineral with analysis, mode of occurrence, and localities having been published in 1948. 3 Sure enough, on examination I found both the Cligga and Bunny minerals to contain a high proportion of tin. Through the interest of Professor K. C. Dunham, Dr. E. A. Vincent of Durham University most readily and kindly undertook the full chemicalexamina- tion of this somewhat remarkable and puzzling material--a quite tricky 1 Now University Demonstrator in the Department of Geology and Mineralogy, University Museum, Oxford. -

Marazion to Porthleven
MARAZION TO PORTHLEVEN This area of Mount’s Bay runs from Marazion in the west to Porthleven in the east. Geological exposures and examples range from metasediments, basic intrusions, granite magmatism and mineralization, contact phenomenon, Variscan tectonics and Quaternary geology. St Michael’s Mount The exposure on the Mount affords one of the best examples of a sheeted greisen vein system hosted mainly in granite in the Cornubian Orefield. Here in the southern portion of granite porphyry hosts a greisen bordered vein system striking east-west up to 50m wide. The granite on the northern side is surrounded by metapelite of Devonian Age. St Michael’s Mount looking west St Michael's Mount comprises a small porphyry granite intrusion (stock) intruded into Devonian mudstones (pelites). The granite, connected to the batholith at depth, is exposed on the southern side (seaward) and is mineralized as it hosts a greisen-bordered sheeted vein system (endogranitic greisen) which carries tin (cassiterite) and tungsten (wolframite) with accessory minerals of lollingite/arsenopyrite (arsenic minerals). The pelites have been thermally metamorphosed to hornfels and in places are cut by granitic veins illustrating the mechanism of igneous emplacement by stoping. Within the granite the greisen veins have a preferred E-W orientation and are closely spaced. The veins show typical space-filling structures with euhedral, vughy and crustiform (minerals deposited in layers) gangue (minerals of no value-quartz etc.) and ore (cassiterite/wolframite). Accessory minerals occurring in the veins are apatite, beryl, topaz and litha micas. The veins are greisen- bordered with white mica and quartz and the borders vary in width and are tensional structures.