Dehydrogenation of Isopropyl Alcohol on Zinc Molybdate (Znmo04)
Total Page:16
File Type:pdf, Size:1020Kb
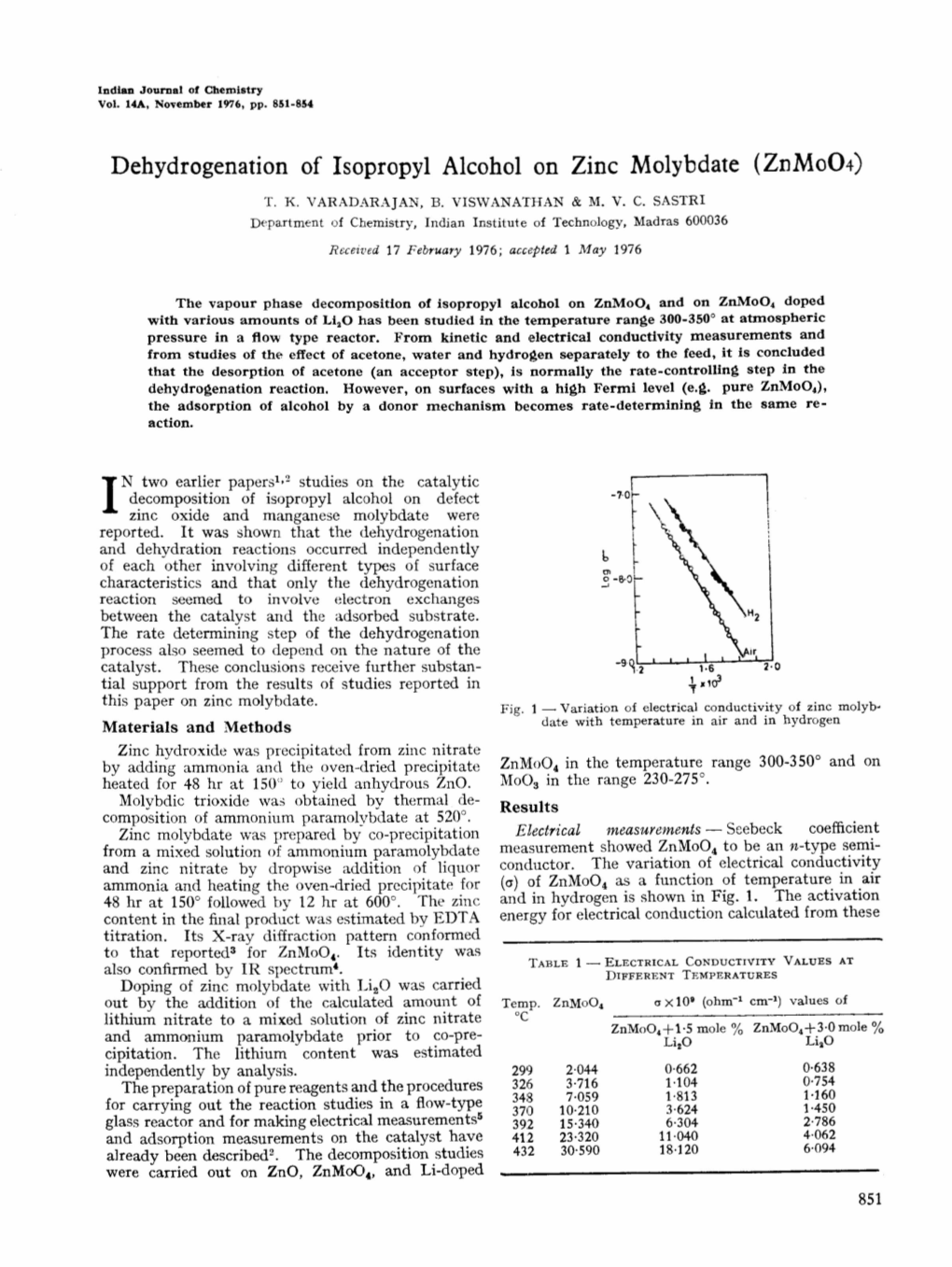
Load more
Recommended publications
-

De Novo Biosynthesis of Terminal Alkyne-Labeled Natural Products
De novo biosynthesis of terminal alkyne-labeled natural products Xuejun Zhu1,2, Joyce Liu2,3, Wenjun Zhang1,2,4* 1Department of Chemical and Biomolecular Engineering, 2Energy Biosciences Institute, 3Department of Bioengineering, University of California, Berkeley, CA 94720, USA. 4Physical Biosciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA. *e-mail:[email protected] 1 Abstract: The terminal alkyne is a functionality widely used in organic synthesis, pharmaceutical science, material science, and bioorthogonal chemistry. This functionality is also found in acetylenic natural products, but the underlying biosynthetic pathways for its formation are not well understood. Here we report the characterization of the first carrier protein- dependent terminal alkyne biosynthetic machinery in microbes. We further demonstrate that this enzymatic machinery can be exploited for the in situ generation and incorporation of terminal alkynes into two natural product scaffolds in E. coli. These results highlight the prospect for tagging major classes of natural products, including polyketides and polyketide/non-ribosomal peptide hybrids, using biosynthetic pathway engineering. 2 Natural products are important small molecules widely used as drugs, pesticides, herbicides, and biological probes. Tagging natural products with a unique chemical handle enables the visualization, enrichment, quantification, and mode of action study of natural products through bioorthogonal chemistry1-4. One prevalent bioorthogonal reaction is -

Dehydrogenation by Heterogeneous Catalysts
Dehydrogenation by Heterogeneous Catalysts Daniel E. Resasco School of Chemical Engineering and Materials Science University of Oklahoma Encyclopedia of Catalysis January, 2000 1. INTRODUCTION Catalytic dehydrogenation of alkanes is an endothermic reaction, which occurs with an increase in the number of moles and can be represented by the expression Alkane ! Olefin + Hydrogen This reaction cannot be carried out thermally because it is highly unfavorable compared to the cracking of the hydrocarbon, since the C-C bond strength (about 246 kJ/mol) is much lower than that of the C-H bond (about 363 kJ/mol). However, in the presence of a suitable catalyst, dehydrogenation can be carried out with minimal C-C bond rupture. The strong C-H bond is a closed-shell σ orbital that can be activated by oxide or metal catalysts. Oxides can activate the C-H bond via hydrogen abstraction because they can form O-H bonds, which can have strengths comparable to that of the C- H bond. By contrast, metals cannot accomplish the hydrogen abstraction because the M- H bonds are much weaker than the C-H bond. However, the sum of the M-H and M-C bond strengths can exceed the C-H bond strength, making the process thermodynamically possible. In this case, the reaction is thought to proceed via a three centered transition state, which can be described as a metal atom inserting into the C-H bond. The C-H bond bridges across the metal atom until it breaks, followed by the formation of the corresponding M-H and M-C bonds.1 Therefore, dehydrogenation of alkanes can be carried out on oxides as well as on metal catalysts. -

Production of Pure Hydrogen by Ethanol Dehydrogenation Abstract
The Future Role of Hydrogen in Petrochemistry and Energy Supply DGMK Conference October 4-6, 2010, Berlin, Germany Production of Pure Hydrogen by Ethanol Dehydrogenation E. Santacesaria, G. Carotenuto, R. Tesser, M. Di Serio University of Naples “Federico II”, Department of Chemistry, Naples, Italy Abstract Hydrogen production from bio-ethanol is one of the most promising renewable processes to generate electricity using fuel cells. In this work, we have studied the production of pure hydrogen as by product of ethanol dehydrogenation reaction. This reaction is promoted by copper based catalysts and according to the catalyst used and the operative conditions gives place to acetaldehyde or ethyl acetate as main products. We studied in particular the performance of a commercial copper/copper chromite catalyst, supported on alumina and containing barium chromate as promoter that has given the best results. By operating at low pressure and temperature with short residence times, acetaldehyde is more selectively produced, while, by increasing the pressure (10-30 bars), the temperature (200-260°C) and the residence time (about 100 (grams hour/mol) of ethanol contact time) the selectivity is shifted to the production of ethyl acetate. However, in both cases pure hydrogen is obtained, as by product, that can easily be separated. Hydrogen obtained in this way is exempt of CO and can be directly fed to fuel cells without any inconvenience. In this work, runs performed in different operative conditions have been reported with the scope to individuate the best conditions. A carrier of H2 6% in N2 has been used. The studied catalyst has also shown a good thermal stability with respect to sintering phenomena, that generally occurs during the -1 -1 dehydrogenation on other copper catalysts. -

Process Technologies and Projects for Biolpg
energies Review Process Technologies and Projects for BioLPG Eric Johnson Atlantic Consulting, 8136 Gattikon, Switzerland; [email protected]; Tel.: +41-44-772-1079 Received: 8 December 2018; Accepted: 9 January 2019; Published: 15 January 2019 Abstract: Liquified petroleum gas (LPG)—currently consumed at some 300 million tonnes per year—consists of propane, butane, or a mixture of the two. Most of the world’s LPG is fossil, but recently, BioLPG has been commercialized as well. This paper reviews all possible synthesis routes to BioLPG: conventional chemical processes, biological processes, advanced chemical processes, and other. Processes are described, and projects are documented as of early 2018. The paper was compiled through an extensive literature review and a series of interviews with participants and stakeholders. Only one process is already commercial: hydrotreatment of bio-oils. Another, fermentation of sugars, has reached demonstration scale. The process with the largest potential for volume is gaseous conversion and synthesis of two feedstocks, cellulosics or organic wastes. In most cases, BioLPG is produced as a byproduct, i.e., a minor output of a multi-product process. BioLPG’s proportion of output varies according to detailed process design: for example, the advanced chemical processes can produce BioLPG at anywhere from 0–10% of output. All these processes and projects will be of interest to researchers, developers and LPG producers/marketers. Keywords: Liquified petroleum gas (LPG); BioLPG; biofuels; process technologies; alternative fuels 1. Introduction Liquified petroleum gas (LPG) is a major fuel for heating and transport, with a current global market of around 300 million tonnes per year. -

Catalytic Hydrogenation and Dehydrogenation Reactions of N-Alkyl-Bis(Carbazole)-Based Hydrogen Storage Materials
catalysts Article Catalytic Hydrogenation and Dehydrogenation Reactions of N-alkyl-bis(carbazole)-Based Hydrogen Storage Materials Joori Jung 1,2,†, Byeong Soo Shin 3,4,†, Jeong Won Kang 4,5,* and Won-Sik Han 1,* 1 Department of Chemistry, Seoul Women’s University, Seoul 01797, Korea; [email protected] 2 REYON Pharmaceutical, Co., Ltd., Anyang 01459, Korea 3 Hyundai Motor Company, Strategy & Technology Division, Ulwang 16082, Korea; [email protected] 4 Department of Chemical and Biological Engineering, Korea University, Seoul 02841, Korea 5 Graduate School of Energy and Environment, Korea University, Seoul 02841, Korea * Correspondence: [email protected] (J.W.K.); [email protected] (W.-S.H.) † These authors contributed equally. Abstract: Recently, there have been numerous efforts to develop hydrogen-rich organic materials because hydrogen energy is emerging as a renewable energy source. In this regard, we designed and prepared four new materials based on N-alkyl-bis(carbazole), 9,90-(2-methylpropane-1,3-diyl)bis(9H- carbazole) (MBC), 9,90-(2-ethylpropane-1,3-diyl)bis(9H-carbazole) (EBC), 9,90-(2-propylpropane-1,3- diyl)bis(9H-carbazole) (PBC), and 9,90-(2-butylpropane-1,3-diyl)bis(9H-carbazole) (BBC), to investi- gate their hydrogen adsorption/hydrogen desorption reactivity depending on the length of the alkyl chain. The gravimetric densities of MBC, EBC, PBC, and BBC were 5.86, 5.76, 5.49, and 5.31 H2 wt %, respectively, again depending on the alkyl chain length. All materials showed complete hydro- genation reactions under ruthenium on an alumina catalyst at 190 ◦C, and complete reverse reactions ◦ and dehydrogenation reactions were observed under palladium on an alumina catalyst at <280 C. -

Acrylonitrile-Butadiene-Styrene Copolymer (ABS)
Eco-profiles of the European Plastics Industry Acrylonitrile-Butadiene-Styrene Copolymer (ABS) A report by I Boustead for Plastics Europe Data last calculated March 2005 abs 1 IMPORTANT NOTE Before using the data contained in this report, you are strongly recommended to look at the following documents: 1. Methodology This provides information about the analysis technique used and gives advice on the meaning of the results. 2. Data sources This gives information about the number of plants examined, the date when the data were collected and information about up-stream operations. In addition, you can also download data sets for most of the upstream operations used in this report. All of these documents can be found at: www.plasticseurope.org. Plastics Europe may be contacted at Ave E van Nieuwenhuyse 4 Box 3 B-1160 Brussels Telephone: 32-2-672-8259 Fax: 32-2-675-3935 abs 2 CONTENTS ABS..................................................................................................................................................4 ECO-PROFILE OF ABS ..............................................................................................................6 abs 3 ABS ABS takes its name from the initial letters of the three immediate precursors: acrylonitrile (CH 2=CH-CN) butadiene (CH 2=C-CH=CH 3) styrene (C 6H5-CH=CH 2) and is a two phase polymer system consisting of a glassy matrix of styrene- acrylonitrile copolymer and the synthetic rubber, styrene-butadiene copolymer. The optimal properties of this polymer are achieved by the appropriate grafting between the glassy and rubbery phases. ABS copolymers have toughness, temperature stability and solvent resistance properties superior to those of high impact polystyrene and are true engineering polymers. They can be formed using all of the common plastics techniques and can also be cold formed using techniques usually associated with metals. -

Catalytic Dehydrogenation of Ethane: a Mini Review of Recent Advances and Perspective of Chemical Looping Technology
catalysts Review Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology Danis Fairuzov, Ilias Gerzeliev, Anton Maximov and Evgeny Naranov * Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences, Leninskiy Prospect, 29, 119991 Moscow, Russia; [email protected] (D.F.); [email protected] (I.G.); [email protected] (A.M.) * Correspondence: [email protected] Abstract: Dehydrogenation processes play an important role in the petrochemical industry. High selectivity towards olefins is usually hindered by numerous side reactions in a conventional crack- ing/pyrolysis technology. Herein, we show recent studies devoted to selective ethylene production via oxidative and non-oxidative reactions. This review summarizes the progress that has been achieved with ethane conversion in terms of the process effectivity. Briefly, steam cracking, catalytic dehydrogenation, oxidative dehydrogenation (with CO2/O2), membrane technology, and chemical looping are reviewed. Keywords: ethylene; ethane; dehydrogenation; cracking; membrane technology; chemical looping 1. Introduction Citation: Fairuzov, D.; Gerzeliev, I.; Ethylene is one of the most critical intermediates in the petrochemical industry and Maximov, A.; Naranov, E. Catalytic the global demand for this chemical is shown in Figure1; it is currently produced through Dehydrogenation of Ethane: A Mini the steam cracking of light hydrocarbon derivatives, mainly ethane and naphtha [1–3]. Review of Recent Advances and Ethylene complexes operate in 57 countries of the world. There are 215 ethylene producing Perspective of Chemical Looping facilities operating in the world. The operators of these complexes are about 100 companies, Technology. Catalysts 2021, 11, 833. and the largest are ExxonMobil, SABIC, DowDuPont [4–6]. -

Locating and Estimating Sources of Styrene
EPA-454/R-93-011 EPA Contract No. 68-D2-0160 Work Assignment No.01 LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF STYRENE Final Report Prepared for: Dallas Safriet Emission Inventory Branch U. S. Environmental Protection Agency Research Triangle Park, North Carolina 27711 Prepared by: Radian Corporation Post Office Box 13000 Research Triangle Park, North Carolina 27709 April 20, 1993 This report has been reviewed by the Office Of Air Quality Planning And Standards, U.S. Environmental Protection Agency, and has been approved for publication. Any mention of trade names or commercial products is not intended to constitute endorsement or recommendation for use. EPA-454/R-93-011 jlh.126 3/9/93 ii TABLE OF CONTENTS Section Page PREFACE ........................iii 1 Purpose of Document ...................1 References for Section 1 ..............4 2 Overview of Document Contents ..............5 3 Background........................7 Nature of Pollutant.................7 Overview of Production and Use ...........9 References for Section 3 ............. 20 4 Emissions from Styrene Production ........... 22 Process Description................ 22 Emissions..................... 31 References for Section 4 ............. 40 5 Emissions from Major Uses of Styrene.......... 42 Polystyrene Production .............. 42 Styrene-Butadiene Copolymer Production ...... 53 Styrene-Acrylonitrile Production ......... 64 Acrylonitrile-Butadiene-Styrene Copolymer Production .................... 72 Unsaturated Polyester Resin Production ...... 81 Miscellaneous -

Ethanol Dehydrogenation: a Reaction Path Study by Means of Temporal Analysis of Products
catalysts Article Ethanol Dehydrogenation: A Reaction Path Study by Means of Temporal Analysis of Products Joachim Pasel 1,*, Johannes Häusler 1 , Dirk Schmitt 1, Helen Valencia 2,3, Maria Meledina 2,3, Joachim Mayer 2,3 and Ralf Peters 1 1 Institute of Energy and Climate Research, IEK-14: Electrochemical Process Engineering, Forschungszentrum Jülich GmbH, 52425 Jülich, Germany; [email protected] (J.H.); [email protected] (D.S.); [email protected] (R.P.) 2 Central Facility for Electron Microscopy (GFE), RWTH Aachen University, 52074 Aachen, Germany; [email protected] (H.V.); [email protected] (M.M.); [email protected] (J.M.) 3 Ernst Ruska-Centre (ER-C) for Microscopy and Spectroscopy with Electrons, Forschungszentrum Jülich GmbH, 52425 Jülich, Germany * Correspondence: [email protected]; Tel.: +49-2461-61-5140 Received: 4 September 2020; Accepted: 4 October 2020; Published: 6 October 2020 Abstract: Conventional fossil fuels such as gasoline or diesel should be substituted in the future by environmentally-friendly alternatives in order to reduce emissions in the transport sector and thus mitigate global warming. In this regard, iso-butanol is very promising as its chemical and physical properties are very similar to those of gasoline. Therefore, ongoing research deals with the development of catalytically-supported synthesis routes to iso-butanol, starting from renewably-generated methanol. This research has already revealed that the dehydrogenation of ethanol plays an important role in the reaction sequence from methanol to iso-butanol. To improve the fundamental understanding of the ethanol dehydrogenation step, the Temporal Analysis of Products (TAP) methodology was applied to illuminate that the catalysts used, Pt/C, Ir/C and Cu/C, are very active in ethanol adsorption. -

The Oxidative Dehydrogenation of N-Octane Using Iron Molybdate
Alkanes, Alkenes and Aromatics: The oxidative dehydrogenation of n-octane using iron molybdate catalysts Thesis submitted in accordance with the requirements of the University of Cardiff for the degree of Doctor of Philosophy by Benjamin Roy Yeo December 2014 Declaration This work has not been submitted in substance for any other degree or award at this or any other university or place of learning, nor is being submitted concurrently in candidature for any degree or other award. Signed ……………………………… (candidate) Date ………………………… STATEMENT 1 This thesis is being submitted in partial fulfillment of the requirements for the degree of PhD. Signed ……………………………… (candidate) Date ………………………… STATEMENT 2 This thesis is the result of my own independent work/investigation, except where otherwise stated. Other sources are acknowledged by explicit references. The views expressed are my own. Signed ……………………………… (candidate) Date ………………………… STATEMENT 3 I hereby give consent for my thesis, if accepted, to be available online in the University’s Open Access repository and for inter-library loan, and for the title and summary to be made available to outside organisations. Signed ……………………………… (candidate) Date ………………………… STATEMENT 4: PREVIOUSLY APPROVED BAR ON ACCESS I hereby give consent for my thesis, if accepted, to be available online in the University’s Open Access repository and for inter-library loans after expiry of a bar on access previously approved by the Academic Standards & Quality Committee. Signed ……………………………… (candidate) Date ………………………… I Acknowledgements First and foremost I would like to thank my Supervisor, Professor Graham Hutchings, for giving me the opportunity to conduct this work and for all his support and advice. I would also like to give a special thank you to the team in the CCI and our collaborators Johnson Matthey, SASOL Technology and the University Kwazulu-Natal for their continuous help, advice, guidance and patience. -

Process Design and Analysis of Ethylene and Propylene
1561 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 61, 2017 The Italian Association of Chemical Engineering Online at www.aidic.it/cet Guest Editors: Petar S Varbanov, Rongxin Su, Hon Loong Lam, Xia Liu, Jiří J Klemeš Copyright © 2017, AIDIC Servizi S.r.l. ISBN 978-88-95608-51-8; ISSN 2283-9216 DOI: 10.3303/CET1761258 Process Design and Analysis of Ethylene and Propylene Manufacturing from Shale Gas Minbo Yanga, Fengqi Youb,* a Northwestern University, Evanston, Illinois, 60208, USA b Cornell University, Ithaca, New York 14853, USA [email protected] In this paper, we develop two novel process designs for manufacturing ethylene and propylene from shale gas. In the first process design, raw shale gas is processed to produce the mixture of ethane-propane, which is then stream co-cracked to produce ethylene and propylene. In the second process design, ethane and propane are produced from shale gas separately; then, ethylene is mainly produced via the steam cracking of ethane, and propylene is mostly manufactured via the dehydrogenation of propane. We also consider a conventional naphtha cracking design, in order to compare the economic and environmental performance of manufacturing ethylene and propylene from shale gas with from naphtha. All process designs are modeled and simulated in Aspen HYSYS to obtain the mass and energy balances. On this basis, we conduct a c omparative techno-economic and environmental analysis. The economic analysis indicates that the two proposed designs results in much lower production costs for ethylene and propylene than the conventional naphtha cracking design. However, in terms of life cycle GHG emissions, manufacturing ethylene and propylene via the naphtha cracking design is more attractive. -

Homogeneously Catalysed Conversion of Aqueous Formaldehyde to H2 and Carbonate
ARTICLE Received 27 Sep 2016 | Accepted 20 Feb 2017 | Published 28 Apr 2017 DOI: 10.1038/ncomms14990 OPEN Homogeneously catalysed conversion of aqueous formaldehyde to H2 and carbonate M. Trincado1, Vivek Sinha2, Rafael E. Rodriguez-Lugo3, Bruno Pribanic1, Bas de Bruin2 & Hansjo¨rg Gru¨tzmacher1 Small organic molecules provide a promising solution for the requirement to store large amounts of hydrogen in a future hydrogen-based energy system. Herein, we report that diolefin–ruthenium complexes containing the chemically and redox non-innocent ligand trop2dad catalyse the production of H2 from formaldehyde and water in the presence of a base. The process involves the catalytic conversion to carbonate salt using aqueous solutions and is the fastest reported for acceptorless formalin dehydrogenation to date. A mechanism supported by density functional theory calculations postulates protonation of a ruthenium hydride to form a low-valent active species, the reversible uptake of dihydrogen by the ligand and active participation of both the ligand and the metal in substrate activation and dihy- drogen bond formation. 1 Department of Chemistry and Applied Biosciences ETH Zu¨rich, Laboratory of Inorganic Chemistry, Wolfgang Pauli Str. 10, Zu¨rich CH-8093, Switzerland. 2 Supramolecular and Homogeneous Catalysis Group, van ’t Hoff Institute for Molecular Sciences (HIMS), University of Amsterdam, Science Park 904, Amsterdam 1098 XH, The Netherlands. 3 Labotatorio de Quı´mica Bioinorga´nica, Centro de Quı´mica, Instituto Venezolano de Investigaciones Cientı´ficas (IVIC), Caracas 1020-A, Venezuela. Correspondence and requests for materials should be addressed to M.T. (email: [email protected]) or to B.d.B.