IS 3508 (1966): Method of Sampling and Test for Ghee [FAD 19: Dairy Products and Equipment]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
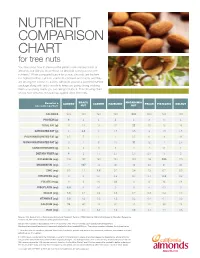
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Sauces Reconsidered
SAUCES RECONSIDERED Rowman & Littlefield Studies in Food and Gastronomy General Editor: Ken Albala, Professor of History, University of the Pacific ([email protected]) Rowman & Littlefield Executive Editor: Suzanne Staszak-Silva ([email protected]) Food studies is a vibrant and thriving field encompassing not only cooking and eating habits but also issues such as health, sustainability, food safety, and animal rights. Scholars in disciplines as diverse as history, anthropol- ogy, sociology, literature, and the arts focus on food. The mission of Row- man & Littlefield Studies in Food and Gastronomy is to publish the best in food scholarship, harnessing the energy, ideas, and creativity of a wide array of food writers today. This broad line of food-related titles will range from food history, interdisciplinary food studies monographs, general inter- est series, and popular trade titles to textbooks for students and budding chefs, scholarly cookbooks, and reference works. Appetites and Aspirations in Vietnam: Food and Drink in the Long Nine- teenth Century, by Erica J. Peters Three World Cuisines: Italian, Mexican, Chinese, by Ken Albala Food and Social Media: You Are What You Tweet, by Signe Rousseau Food and the Novel in Nineteenth-Century America, by Mark McWilliams Man Bites Dog: Hot Dog Culture in America, by Bruce Kraig and Patty Carroll A Year in Food and Beer: Recipes and Beer Pairings for Every Season, by Emily Baime and Darin Michaels Celebraciones Mexicanas: History, Traditions, and Recipes, by Andrea Law- son Gray and Adriana Almazán Lahl The Food Section: Newspaper Women and the Culinary Community, by Kimberly Wilmot Voss Small Batch: Pickles, Cheese, Chocolate, Spirits, and the Return of Artisanal Foods, by Suzanne Cope Food History Almanac: Over 1,300 Years of World Culinary History, Cul- ture, and Social Influence, by Janet Clarkson Cooking and Eating in Renaissance Italy: From Kitchen to Table, by Kath- erine A. -

Olive Oil Jars Left Behind By
live oil jars left behind by the ancient Greeks are testament to our centuries- old use of cooking oil. Along with salt and pepper, oil Oremains one of the most important and versatile tools in your kitchen. It keeps food from sticking to pans, adds flavor and moisture, and conducts the heat that turns a humble stick of potato into a glorious french fry. Like butter and other fats, cooking oil also acts as a powerful solvent, unleashing fat-soluble nutrients and flavor compounds in everything from tomatoes and onions to spices and herbs. It’s why so many strike recipes begin with heating garlic in oil rather than, say, simmering it in water. The ancient Greeks didn’t tap many cooking oils. (Let’s see: olive oil, olive oil, or—ooh, this is exciting!—how about olive oil?) But you certainly can. From canola to safflower to grapeseed to walnut, each oil has its own unique flavor (or lack thereof), aroma, and optimal cooking temperature. Choosing the right kind for the task at hand can save you money, boost your health, and improve your cooking. OK, so you probably don’t stop to consider your cooking oil very often. But there’s a surprising amount to learn about What’s this? this liquid gold. BY VIRGINIAWILLIS Pumpkin seed oil suspended in corn oil—it looks like a homemade Lava Lamp! 84 allrecipes.com PHOTOS BY KATE SEARS WHERE TO store CANOLA OIL GRAPESEED OIL are more likely to exhibit the characteristic YOUR OIL flavor and aroma of their base nut or seed. -

Some of the Factors Influencing the Growth of Molds in Butter Harold Macy Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1929 Some of the factors influencing the growth of molds in butter Harold Macy Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agriculture Commons, and the Food Science Commons Recommended Citation Macy, Harold, "Some of the factors influencing the growth of molds in butter " (1929). Retrospective Theses and Dissertations. 14244. https://lib.dr.iastate.edu/rtd/14244 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the miaofilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. in the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Al.so, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections vwth small overiaps. -
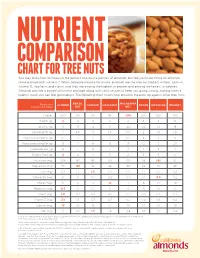
Chart for Tree Nuts
NUTRIENT COMPARISON CHART FOR TREE NUTS You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the highest in protein and among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a healthy snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT Calories 1602 190 160 180 200 200 160 190 Protein (g) 6 4 4 4 2 3 6 4 Total Fat (g) 14 19 13 17 22 20 13 18 Saturated Fat (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 Polyunsaturated Fat (g) 3.5 7 2 2 0.5 6 4 13 Monounsaturated Fat (g) 9 7 8 13 17 12 7 2.5 Carbohydrates (g) 6 3 9 5 4 4 8 4 Dietary Fiber (g) 4 2 1 3 2 3 3 2 Potassium (mg) 208 187 160 193 103 116 285 125 Magnesium (mg) 77 107 74 46 33 34 31 45 Zinc (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 Vitamin B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 Folate (mcg) 12 6 20 32 3 6 14 28 Riboflavin (mg) 0.3 0 0.1 0 0 0 0.1 0 Niacin (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 Vitamin E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.6 0.2 Calcium (mg) 76 45 13 32 20 20 30 28 Iron (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Cooking Oil Facts
Cooking Oil Facts As you enter a department store, you behold an array of cooking oils sporting all types of jargon on the packaging -- saturated fats, unsaturated fats, refined, filtered, ricebran oil, vanaspati, etc. Confused already? With so much variety and so many brands flooding the market today, buying the right cooking oil can prove a tough task. Different oils fill different needs - for health, taste and cooking. For good health, our bodies need a variety of healthy fats that are found naturally in different oils. When cooking, it's essential to know which oils are best for baking, sautéing and frying and which are healthiest used raw. Why have Oil (fats)? Contrary to popular belief, fat is actually a valuable part of one's diet, allowing people to absorb nutrients that require fat in order to metabolize in the body. Natural fats contain varying ratios of three types of fats: saturated, monounsaturated and polyunsaturated. • Saturated fats are hard at room temperature. They're stable, resist oxidation, and are found primarily in meat, dairy, palm and coconut oil. • Polyunsaturated fats are liquid at room temperature and the least stable. They oxidize easily and are found in seafood corn, safflower, soybean, and sunflower oils. • Monounsaturated fats are more stable than polyunsaturated fats. They're found in canola, nut and olive oils. It is recommended to limit saturated fats in the diet due to their association with cardiovascular disease. Also, you should try to rely more on monounsaturated than polyunsaturated fats. What are the varieties of Oil available in the market? Choosing which oil should be used in cooking is a big issue and concern for many people because of the fat and cholesterol contents of cooking oil. -

Nutrition Handout N09: What Are the Types of Fat?
N09 What Are the Types of Fat? Most foods contain several different kinds of fat. Some are better for your health than others. It is wise to choose healthier types of fat, and enjoy them in moderation. Keep in mind that even healthier fats contain calories and should be used sparingly for weight management. Here is some information about healthy and harmful dietary fats. The four major types of fats are: • Monounsaturated fats • Polyunsaturated fats • Saturated fats • Trans fats Monounsaturated and polyunsaturated fats are known as “healthy fats” because they are good for your heart, cholesterol levels, and overall health. These fats tend to be “liquid” at room temperature. Consider beneficial polyunsaturated fats containing Omega-3 fatty acids found in fatty fish, flaxseed, and walnuts. www.move.va.gov Nutrition Handouts • N09 Version 5.0 Page 1 of 3 Healthy Dietary Fats Monounsaturated Fat Polyunsaturated Fat Olive oil Soybean oil Canola oil Corn oil Sunflower oil Safflower oil Peanut oil Walnuts Olives Sunflower, sesame, and pumpkin seeds; flaxseed Nuts (almonds, peanuts, hazelnuts, Fatty fish (salmon, tuna, mackerel, herring, macadamia nuts, pecans, cashews) trout, anchovies, sardines, and eel) Avocados Soymilk Peanut butter Tofu Tips for increasing healthy fats in your diet: • Cook with olive oil. • Plan snacks of nuts or olives. • Eat more avocados. • Dress your own salads instead of using commercial dressings. Saturated fats and trans fats are known as the “harmful fats.” They increase your risk of disease and elevate cholesterol. Saturated fats tend to be solid at room temperature, but they are also found in liquid tropical oils (palm and coconut). -

Fats Ebook Feb 02.Pdf
2 DRHYMAN.COM Contents Contents INTRODUCTION ................................. 8 PART I ........................................... 11 Dietary Fats: The Good, Bad and the Ugly ............................................ 11 Fatty Acids ............................................................................................ 11 Saturated Fat ........................................................................................ 12 Polyunsaturated Fats ............................................................................ 14 Essential Fatty Acids 101- Omega-3 and Omega-6 ............................... 14 The Beneficial Omega-6 Fatty Acid: GLA ............................................... 16 How Fatty Acids Affect Brain Health ..................................................... 17 Omega-7 Fatty Acids ............................................................................ 18 Monounsaturated Fat ............................................................................ 18 Trans Fats ............................................................................................. 20 Trans Fats and Health ........................................................................... 21 Toxins in Fat .......................................................................................... 22 A Case for Organic ................................................................................ 23 DRHYMAN.COM 3 PART II .......................................... 24 Animal Fats ....................................................................... -

Talking About Trans Fat: What You Need to Know
FOODFACTS From the U.S. Food and Drug Administration Talking About Trans Fat What You Need to Know Trans Fat at-a-Glance Fats in Your Diet Trans fat is a specific type of fat that is formed when liquid oils are turned Fat is a major source of energy for the body, into solid fats, such as shortening or stick margarine. During this process and aids in the absorption of vitamins A, — called hydrogenation — hydrogen is added to vegetable oil to increase D, E, and K, as well as carotenoids. Both the shelf life and flavor stability of foods. The result of the process is animal- and plant-derived food products trans fat. contain fat, and fat is important for proper growth, development, and maintenance of • fat can be found in many of the same foods as saturated fat, such Trans good health. as vegetable shortenings, some margarines, crackers, candies, cookies, snack foods, fried foods, baked goods, and other processed foods made However, experts recommend that you get with partially hydrogenated vegetable oils. about one third or less of your calories (i.e., between 20 and 35 percent of calories) • Also known as trans fatty acids, trans fat can be found naturally in some from fat. foods — such as animal-based foods like milk, milk products, and meat. • As a food ingredient, fat provides flavor, As a consumer, the most important thing to know about trans fat is that it consistency, and stability — and helps you behaves like saturated fat in the body by raising low-density lipoprotein feel full. -

Breakfast Glycaemic Response in Patients with Type 2 Diabetes: Effects of Bedtime Dietary Carbohydrates
European Journal of Clinical Nutrition (1999) 53, 706±710 ß 1999 Stockton Press. All rights reserved 0954±3007/99 $15.00 http://www.stockton-press.co.uk/ejcn Breakfast glycaemic response in patients with type 2 diabetes: Effects of bedtime dietary carbohydrates M Axelsen1*, R Arvidsson Lenner 2,PLoÈnnroth1 and U Smith 1 1The Lundberg Laboratory for Diabetes Research, Department of Internal Medicine, Sahlgrenska University Hospital, S-413 45 GoÈteborg, Sweden; and 2Department of Clinical Nutrition, Department of Internal Medicine, Sahlgrenska University Hospital, GoÈteborg University, Sweden Objectives: Bedtime carbohydrate (CHO) intake in patients with type-2 diabetes may improve glucose tolerance at breakfast the next morning. We examined the `overnight second-meal effect' of bedtime supplements containing `rapid' or `slow' CHOs. Design: Randomized cross-over study with three test-periods, each consisting of two days on a standardized diet, followed by a breakfast tolerance test on the third morning. Setting: The Lundberg Laboratory for Diabetes Research, Sahlgrenska University Hospital, GoÈteborg, Sweden. Subjects: Sixteen patients with type 2 diabetes on oral agents and=or diet. Interventions: Two different bedtime (22.00 h) CHO supplements (0.46 g available CHO=kg body weight) were compared to a starch-free placebo (`normal' food regimen). The CHOs were provided as uncooked cornstarch (slow-release CHOs) or white bread (rapid CHOs). Results: On the mornings after different bedtime meals we found similar fasting glucose, insulin, free fatty acid and lactate levels. However, the glycaemic response after breakfast was 21% less after uncooked cornstarch compared to placebo ingestion at bedtime (406Æ 46 vs 511Æ 61 mmol min 171, P < 0.01). -

Butter and Ghee 20
NEWSLETTER20 EGERTON UNIVERSITY Transforming Lives through Quality Education DIVISION OF RESEARCH & EXTENSION Making Butter and Ghee at Home What is Butter and Ghee? Butter and ghee are made from fat from whole milk. Butter contains 80% fat while ghee contains 99.9% fat. Ghee is clarified butter that has been cooked longer to remove all the moisture and the milk solids are browned in the fat and then strained out. Why Butter and Ghee? Both butter and ghee are rich in proteins, fat soluble vitamins and fatty acids which are important to maintain good health. They can also be prepared at home using simple processes and used for cooking. Procedure of Making Butter and Homemade butter Ghee 1. Boil milk and let it cool for 4-5 hours. 2. Collect the cream and place in a blender. 3. Add 2 cups chilled water and run the blender for 2-3 minutes. Leave a gap of a few seconds then run again. 4. After 10-15 minutes of whipping, the butter will collect at the top. The liquid remaining is buttermilk. Collect the butter with a ladle and place it in a bowl of chilled water. 5. Wash the butter 2-3 times in chilled water until the water is clear. 6. Press and remove as much water as possible and store the butter in a container and keep in the refrigerator. 7. Heat the butter in a heavy bottomed pan over medium heat. 8. When the butter melts, reduce the heat to low. It will start boiling with a lot of bubbles and later foam at the top. -

Butter, Margarine, Vegetable Oils, and Olive Oil in the Average Polish Diet
nutrients Article Butter, Margarine, Vegetable Oils, and Olive Oil in the Average Polish Diet Hanna Górska-Warsewicz * , Krystyna Rejman , Wacław Laskowski and Maksymilian Czeczotko Department of Food Market and Consumer Research, Institute of Human Nutrition Sciences, Warsaw University of Life Sciences, 02-787 Warsaw, Poland; [email protected] (K.R.); [email protected] (W.L.); [email protected] (M.C.) * Correspondence: [email protected]; Tel.: +48-22-5937144 Received: 13 November 2019; Accepted: 27 November 2019; Published: 3 December 2019 Abstract: The main aim of this study was to identify the sources of energy and 25 nutrients in fats and oils in the average Polish diet. We analyzed energy, total fat, saturated fatty acids (SFAs), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), cholesterol, protein, carbohydrates, nine minerals, and nine vitamins. We included five sub-groups: butter, vegetable oils, margarine and other hydrogenated vegetable fats, olive oil, and other animal fats. The basis for our analysis was data from the 2016 household budget survey, conducted on a representative sample of the Polish population (36,886 households, n = 99,230). We used the cluster analysis to assess the impact of socio-demographic and economic factors on the volume of fats and oil consumption and on the share of particular products in the supply of energy and nutrients. Our findings indicated that fats and oils contributed 32.9% of the total fat supply, which placed these products in first position among main food groups. Meat and its products ranked second (30.8%) in the total fat supply, while milk and dairy products, including cream (13.4%), were the third food group.