Expression of Type XVI Collagen in Human Skin Fibroblasts: Enhanced Expression in Fibrotic Skin Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Novel Mechanism of Metastasis in Postpartum Breast Cancer
WEANING-INDUCED LIVER INVOLUTION: A NOVEL MECHANISM OF METASTASIS IN POSTPARTUM BREAST CANCER By Erica T. Goddard A THESIS/DISSERTATION Presented to the Cancer Biology Program & the Oregon Health & Science University School of Medicine in partial fulfillment of the requirements for the degree of Doctor of Philosophy January 2017 School of Medicine Oregon Health & Science University CERTIFICATE OF APPROVAL ______________________________ This is to certify that the PhD dissertation of Erica Thornton Goddard has been approved _________________________________ Pepper J. Schedin, PhD, Mentor/Advisor _________________________________ Virginia F. Borges, MD, MMSc, Clinical Mentor/Advisor _________________________________ Melissa H. Wong, PhD, Oral Exam Committee Chair _________________________________ Lisa Coussens, PhD, Member _________________________________ Amanda W. Lund, PhD, Member _________________________________ Caroline Enns, PhD, Member _________________________________ Willscott E. Naugler, MD, Member Table of Contents List of Figures ........................................................................................................................... iv List of Tables ............................................................................................................................ vii List of abbreviations ............................................................................................................ viii Acknowledgements ............................................................................................................. -

Increased Expression of Several Collagen Genes Is Associated With
Journal of Cancer 2016, Vol. 7 1295 Ivyspring International Publisher Journal of Cancer 2016; 7(10): 1295-1310. doi: 10.7150/jca.15371 Research Paper Increased Expression of Several Collagen Genes is Associated with Drug Resistance in Ovarian Cancer Cell Lines Radosław Januchowski1, Monika Świerczewska1, Karolina Sterzyńska1, Karolina Wojtowicz1, Michał Nowicki1, Maciej Zabel1,2 1. Department of Histology and Embryology, Poznań University of Medical Sciences, Poland; 2. Department of Histology and Embryology, Wroclaw Medical University, Poland. Corresponding author: Radosław Januchowski, mailing address: Department of Histology and Embryology, Poznan University of Medical Sciences, Święcickiego 6 St., Poznań, post code 61-781, phone number +48618546419, fax number +48 618546440, email address: [email protected]. © Ivyspring International Publisher. Reproduction is permitted for personal, noncommercial use, provided that the article is in whole, unmodified, and properly cited. See http://ivyspring.com/terms for terms and conditions. Received: 2016.02.25; Accepted: 2016.05.23; Published: 2016.06.25 Abstract Ovarian cancer is the most lethal gynaecological cancer. The main reason for the high mortality among ovarian cancer patients is the development of drug resistance. The expression of collagen genes by cancer cells can increase drug resistance by inhibiting the penetration of the drug into the cancer tissue as well as increase apoptosis resistance. In this study, we present data that shows differential expression levels of collagen genes and proteins in cisplatin- (CIS), paclitaxel- (PAC), doxorubicin- (DOX), topotecan- (TOP), vincristine- (VIN) and methotrexate- (MTX) resistant ovarian cancer cell lines. Quantitative real-time polymerase chain reactions were performed to determine the mRNA levels. Protein expression was detected using Western blot and immunocytochemistry assays. -

Effect of Selenocystine on Gene Expression Profiles in Human Keloid
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Genomics 97 (2011) 265–276 Contents lists available at ScienceDirect Genomics journal homepage: www.elsevier.com/locate/ygeno Effect of selenocystine on gene expression profiles in human keloid fibroblasts Bruna De Felice a,⁎, Corrado Garbi b, Robert R. Wilson a, Margherita Santoriello b, Massimo Nacca a a Department of Life Sciences, University of Naples II, Via Vivaldi 43, 81100 Caserta, Italy b Department of Cellular and Molecular Biology and Pathology, University of Naples “Federico II”, Naples, Italy article info abstract Article history: In this study, selenocystine, a nutritionally available selenoamino acid, was identified for the first time as a Received 15 October 2010 novel agent with anti proliferative activity on human keloids. Accepted 24 February 2011 The 20 μM concentration after 48 h treatment used here was the most effective to reduce keloid fibroblast Available online 1 March 2011 growth. We analyzed the gene expression profile of selenocystine treatment response in keloid fibroblasts by the microarray system to characterize the effects of selenocystine on human keloids. Keywords: The major alterations in keloid fibroblasts following selenocystine exposure included up-regulation of the Keloids Microarray genes encoding cell death and transcription factors. Prominent down-regulation of genes involved in Selenocystine development, cell adhesion and cytoskeleton, as well as extra cellular matrix genes, usually strongly up- Gene expression regulated in keloids, resulted following selenocystine exposure. The range of the down-regulated genes and the degree of the decreased expression appeared to be correlated with the degree of the morphological alterations in selenocystine treated keloids. -

Cell-Deposited Matrix Improves Retinal Pigment Epithelium Survival on Aged Submacular Human Bruch’S Membrane
Retinal Cell Biology Cell-Deposited Matrix Improves Retinal Pigment Epithelium Survival on Aged Submacular Human Bruch’s Membrane Ilene K. Sugino,1 Vamsi K. Gullapalli,1 Qian Sun,1 Jianqiu Wang,1 Celia F. Nunes,1 Noounanong Cheewatrakoolpong,1 Adam C. Johnson,1 Benjamin C. Degner,1 Jianyuan Hua,1 Tong Liu,2 Wei Chen,2 Hong Li,2 and Marco A. Zarbin1 PURPOSE. To determine whether resurfacing submacular human most, as cell survival is the worst on submacular Bruch’s Bruch’s membrane with a cell-deposited extracellular matrix membrane in these eyes. (Invest Ophthalmol Vis Sci. 2011;52: (ECM) improves retinal pigment epithelial (RPE) survival. 1345–1358) DOI:10.1167/iovs.10-6112 METHODS. Bovine corneal endothelial (BCE) cells were seeded onto the inner collagenous layer of submacular Bruch’s mem- brane explants of human donor eyes to allow ECM deposition. here is no fully effective therapy for the late complications of age-related macular degeneration (AMD), the leading Control explants from fellow eyes were cultured in medium T cause of blindness in the United States. The prevalence of only. The deposited ECM was exposed by removing BCE. Fetal AMD-associated choroidal new vessels (CNVs) and/or geo- RPE cells were then cultured on these explants for 1, 14, or 21 graphic atrophy (GA) in the U.S. population 40 years and older days. The explants were analyzed quantitatively by light micros- is estimated to be 1.47%, with 1.75 million citizens having copy and scanning electron microscopy. Surviving RPE cells from advanced AMD, approximately 100,000 of whom are African explants cultured for 21 days were harvested to compare bestro- American.1 The prevalence of AMD increases dramatically with phin and RPE65 mRNA expression. -

UNIVERSITY of CALIFORNIA, SAN DIEGO the Role of Collagen on The
UNIVERSITY OF CALIFORNIA, SAN DIEGO The role of collagen on the structural response of dermal layers in mammals and fish A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Materials Science and Engineering by Vincent Robert Sherman Committee in charge: Professor Marc A. Meyers, Chair Professor Shengqiang Cai Professor Xanthippi Markenscoff Professor Joanna McKittrick Professor Jan Talbot 2016 Copyright Vincent Robert Sherman, 2016 All rights reserved The Dissertation of Vincent Robert Sherman is approved, and is acceptable in quality and form for publication on microfilm and electronically: ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ Chair University of California, San Diego 2016 iii TABLE OF CONTENTS SIGNATURE PAGE ......................................................................................................... iii TABLE OF CONTENTS ................................................................................................... iv LIST OF FIGURES ........................................................................................................... ix LIST OF TABLES ........................................................................................................... xiii ACKNOWLEDGEMENTS ............................................................................................ -

Outcome of Split Thickness Skin Grafts on Excised Burns with Different Dermal Compositions
Department of Plastic Surgery and Department of Pharmacology Faculty of Medicine University of Helsinki Finland Outcome of split thickness skin grafts on excised burns with different dermal compositions Heli Lagus Academic dissertation To be presented, with the permission of the Faculty of Medicine of the University of Helsinki, for public examination Porthania lecture hall P674, Helsinki, on June 26th 2020, at 12 o’clock noon. Helsinki 2020 Supervised by Professor Jyrki Vuola, M.D., Ph.D. Department of Plastic Surgery Helsinki Burn Centre Helsinki University Hospital and University of Helsinki Finland Docent Esko Kankuri, M.D., Ph.D. Department of Pharmacology University of Helsinki Finland Reviewed by Julian Dye, MA (Oxon), Ph.D. (Lon) CBiol, FRSB Institute of Biomedical Engineering University of Oxford United Kingdom Docent Ilkka Kaartinen, M.D., Ph.D. Department of Plastic Surgery Tampere University Hospital Finland Opponent Professor Esther Middelkoop, Ph.D. in biochemistry Plastic, Reconstructive and Hand Surgery Amsterdam UMC - Vrije Universiteit Amsterdam The Netherlands The Faculty of Medicine uses the Urkund system (plagiarism recognition) to examine all doctoral dissertations. ISBN: 978-951-51-6066-9 (print) ISBN: 978-951-51-6067-6 (on-line) University Printing House Helsinki 2020 To my family 3 Table of Contents List of original publications........................................................................................................... 6 Abbreviations ............................................................................................................................... -
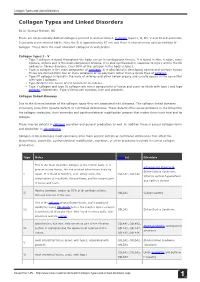
Collagen Types and Linked Disorders
Collagen Types and Linked Disorders Collagen Types and Linked Disorders By Dr Ananya Mandal, MD There are 29 genetically distinct collagens present in animal tissues. Collagen types I, II, III, V and XI self-assemble into D-periodic cross-striated fibrils. Here the D is approximately 67 nm and there is characteristic axial periodicity of collagen. These form the most abundant collagens in vertebrates. Collagen types I - V • Type I collagen is found throughout the body except in cartilaginous tissues. It is found in skin, tendon, vascular, ligature, organs and is the main component of bone. It is also synthesized in response to injury and in the fibrous nodules in fibrous diseases. Over 90% of the collagen in the body is type I. • Type II collagen is the main component of cartilage. It is also found in developing cornea and vitreous humour. These are formed from two or more collagens or co-polymers rather than a single type of collagen. • Type III collagen is found in the walls of arteries and other hollow organs and usually occurs in the same fibril with type I collagen. • Type IV forms the bases of cell basement membrane • Type V collagen and type XI collagen are minor components of tissue and occur as fibrils with type I and type II collagen respectively. Type V forms cell surfaces, hair and placenta. Collagen linked diseases Due to the diverse location of the collagen types they are associated with diseases. The collagen linked diseases commonly arise from genetic defects or nutritional deficiencies. These defects often cause problems in the biosynthesis of the collagen molecules, their assembly and posttranslational modification process that makes them their final end form of collagen. -

Nature's Solution to a Hard-Soft Interface Lara Angelika Dorothée Kuntz
Technische Universität München Fakultät für Medizin The enthesis: nature’s solution to a hard-soft interface Lara Angelika Dorothée Kuntz Vollständiger Abdruck der von der Fakultät für Medizin der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) genehmigten Dissertation. Vorsitzender: Prof. Dr. Gil G. Westmeyer Prüfende der Dissertation: 1. apl. Prof. Dr. Rainer Burgkart 2. Prof. Dr. Andreas Bausch 3. Prof. Dr. Felix Eckstein Die Dissertation wurde am 10.05.2017 bei der Technischen Universität München eingereicht und durch die Fakultät für Medizin am 21.02.2018 angenommen. Prefix This dissertation was part of the biomaterials focus group project “The enthesis: Nature’s solution to a hard-soft junction“ funded by the International Graduate School of Science and Engineering (IGSSE) of the Technical University of Munich. It was a collaborative project between the Technical University of Munich Clinics of Orthopedics/Sports Orthopedics and the Chair of Cellular Biophysics. Herewith I would like to thank everybody who contributed to this thesis directly or indirectly. It was a very lively, interesting time and many have contributed to it being fun and productive. I thank my advisors Professor Rainer Burgkart and Professor Andreas Bausch for supervi- sion and guidance. Thank you, Rainer, for keeping me motivated to dig deep into the Achilles heel with your positive attitude and your passion for the enthesis. Thank you, Andreas, for your scientific advice and for important lessons for life. Thank you both for giving me the freedom to develop project ideas on my own, yet giving me guidance and direction when I felt stuck. -

Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions
Downloaded from http://cshperspectives.cshlp.org/ on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions Richard O. Hynes and Alexandra Naba Howard Hughes Medical Institute, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 Correspondence: [email protected] Completion of genome sequences for many organisms allows a reasonably complete definition of the complement of extracellular matrix (ECM) proteins. In mammals this “core matrisome” comprises 300 proteins. In addition there are large numbers of ECM- modifying enzymes, ECM-binding growth factors, and other ECM-associated proteins. These different categories of ECM and ECM-associated proteins cooperate to assemble and remodel extracellular matrices and bind to cells through ECM receptors. Togetherwith recep- tors for ECM-bound growth factors, they provide multiple inputs into cells to control survival, proliferation, differentiation, shape, polarity, and motility of cells. The evolution of ECM pro- teins was key in the transition to multicellularity, the arrangement of cells into tissue layers, and the elaboration of novel structures during vertebrate evolution. This key role of ECM is reflected in the diversity of ECM proteins and the modular domain structures of ECM proteins both allow their multiple interactions and, during evolution, development of novel protein architectures. he term extracellular matrix (ECM) means make up basement membranes. Biochemistry Tsomewhat different things to different of native ECM was, and still is, impeded by people (Hay 1981, 1991; Mecham 2011). Light the fact that the ECM is, by its very nature, and electron microscopy show that extracellular insoluble and is frequently cross-linked. -

Corneal Collagen Fibril Structure in Three Dimensions: Structural Insights Into Fibril Assembly, Mechanical Properties, and Tissue Organization
Corneal collagen fibril structure in three dimensions: Structural insights into fibril assembly, mechanical properties, and tissue organization David F. Holmes*, Christopher J. Gilpin*, Clair Baldock*, Ulrike Ziese†, Abraham J. Koster†, and Karl E. Kadler*‡ *Wellcome Trust Centre for Cell-Matrix Research, School of Biological Sciences, University of Manchester, Stopford Building 2.205, Oxford Road, Manchester M13 9PT, United Kingdom; and †Department of Molecular Cell Biology, Utrecht University, Padualaan 8, 3584 CH Utrecht, The Netherlands Communicated by Darwin J. Prockop, Tulane University, New Orleans, LA, March 26, 2001 (received for review November 3, 2000) The ability of the cornea to transmit light while being mechanically substructure or a crystalline packing of collagen molecules in a resilient is directly attributable to the formation of an extracellular sheet structure (3–5). A five-stranded microfibrillar model was matrix containing orthogonal sheets of collagen fibrils. The de- proposed originally by Smith (6) and then developed by Piez and tailed structure of the fibrils and how this structure underpins the Trus (7, 8) on the basis of sequence regularities and x-ray mechanical properties and organization of the cornea is under- diffraction data. Recent analysis of synchrotron x-ray diffraction stood poorly. In this study, we used automated electron tomog- from rat tail tendon has yielded the best agreement with a raphy to study the three-dimensional organization of molecules in microfibril substructure (9). corneal collagen fibrils. The reconstructions show that the collagen Corneal collagen fibrils are heterotypic structures composed molecules in the 36-nm diameter collagen fibrils are organized into of type I collagen molecules coassembled along with those of microfibrils (Ϸ4-nm diameter) that are tilted by Ϸ15° to the fibril type V collagen (10). -
Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions
Downloaded from http://cshperspectives.cshlp.org/ on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions Richard O. Hynes and Alexandra Naba Howard Hughes Medical Institute, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 Correspondence: [email protected] Completion of genome sequences for many organisms allows a reasonably complete definition of the complement of extracellular matrix (ECM) proteins. In mammals this “core matrisome” comprises 300 proteins. In addition there are large numbers of ECM- modifying enzymes, ECM-binding growth factors, and other ECM-associated proteins. These different categories of ECM and ECM-associated proteins cooperate to assemble and remodel extracellular matrices and bind to cells through ECM receptors. Togetherwith recep- tors for ECM-bound growth factors, they provide multiple inputs into cells to control survival, proliferation, differentiation, shape, polarity, and motility of cells. The evolution of ECM pro- teins was key in the transition to multicellularity, the arrangement of cells into tissue layers, and the elaboration of novel structures during vertebrate evolution. This key role of ECM is reflected in the diversity of ECM proteins and the modular domain structures of ECM proteins both allow their multiple interactions and, during evolution, development of novel protein architectures. he term extracellular matrix (ECM) means make up basement membranes. Biochemistry Tsomewhat different things to different of native ECM was, and still is, impeded by people (Hay 1981, 1991; Mecham 2011). Light the fact that the ECM is, by its very nature, and electron microscopy show that extracellular insoluble and is frequently cross-linked. -
Method for Making a Biofabricated Material Containing Collagen Fibrils
(19) TZZ¥ Z_T (11) EP 3 205 666 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 16.08.2017 Bulletin 2017/33 C07K 14/78 (2006.01) D06N 3/00 (2006.01) D01F 4/00 (2006.01) D01F 11/02 (2006.01) (21) Application number: 17156361.2 (22) Date of filing: 15.02.2017 (84) Designated Contracting States: • Cassingham, Darryl Miles AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Dorset DT9 6RF (GB) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (72) Inventors: Designated Extension States: • Purcell, Brendan Patrick BA ME Brooklyn, NY 11225 (US) Designated Validation States: • Williamson, David Thomas MA MD Landenberg, PA 19350 (US) • Marga, Francoise Suzanne (30) Priority: 15.02.2016 US 201662295435 P Brooklyn, NY 11209 (US) • Shofer, Susan J. (71) Applicants: Brooklyn, NY 11231 (US) • Purcell, Brendan Patrick • Cassingham, Darryl Miles Brooklyn, NY 11225 (US) Dorset DT9 6RF (GB) • Williamson, David Thomas Landenberg, PA 19350 (US) (74) Representative: Brevalex • Marga, Francoise Suzanne 56, Boulevard de l’Embouchure Brooklyn, NY 11209 (US) B.P. 27519 • Shofer, Susan J. 31075 Toulouse Cedex 2 (FR) Brooklyn, NY 11231 (US) (54) METHOD FOR MAKING A BIOFABRICATED MATERIAL CONTAINING COLLAGEN FIBRILS (57) Described herein is a method for producing a techniques or by chemical synthesis. The collagen or col- biofabricated material from collagen or collagen-like pro- lagen-like proteins are fibrillated, crosslinked, dehydrat- teins. The collagen or collagen-like proteins are isolated ed and lubricated thus forming the biofabricated material from animal sources or produced by recombinant DNA having a substantially uniform network of collagen fibrils.