How Do Fireworks Work?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification, Chemistry, and Behavior of Seal Bombs Used to Control
f MARCH 1990 h IDENTIFICATION, CHEMISTRY, AND BEHAVIOR OF SEAL BOMBS USED TO CONTROL DOLPHINS IN THE YELLOWFIN TUNA PURSE-SEINE FISHERY IN THE EASTERN TROPICAL PACIFIC: POTENTIAL HAZARDS By Albert C. Myrick Jr. Martin Fink Cheryl B. Glick ADMINISTRATIVE REPORT LJ-90-08 f This administrative Report is issued as an informal document to ensure prompt dissemination of preliminary results, interim reports and special studies. We recommend that it not be abstracted or cited. 5H // Sic 2 ft,o. 90-0? C. ^ IDENTIFICATION, CHEMISTRY, AND BEHAVIOR OF SEAL BOMBS USED TO CONTROL DOLPHINS IN THE YELLOWFIN TUNA PURSE-SEINE FISHERY IN THE EASTERN TROPICAL PACIFIC: POTENTIAL HAZARDS By 12 1 Albert C. Myrick Jr., Martin Fink, and Cheryl B. Glick 1. Southwest Fisheries Center, National Marine Fisheries Service, P.O. Box 271, La Jolla, CA 92038 2. San Diego County Sheriff's Dept., Crime Laboratory, 3520 Kurtz Street, San Diego, CA 92110 LIBRARY March 1990 FEB 28 2008 National oceanic & Atmospheric Administration U.S. Dept, of Commerce ADMINISTRATIVE REPORT LJ-90-08 CONTENTS Page ABSTRACT...................................................... 1 INTRODUCTION.................................................. 1 METHODS AND MATERIALS........................................ 3 RESULTS Description.............................................. 4 Chemical Analysis and Apparent TNT Equivalents........ 5 Charge-Weights and Relative Strengths.................. 7 Behavior of Units Detonated............................. 7 Relative Strengths Based on Combined Characteristics.. -

IED and Explosive Effects Fundamentals DHS-MITG-253 Version 4
IED and Explosive Effects Fundamentals DHS-MITG-253 Version 4 Office for Bombing Prevention IED and Explosive Effects Fundamentals Objectives At the end of this module, participants will be able to: 1) Define the term “Improvised Explosive Device” (IED) 2) Identify the components of an IED 3) Describe blast, thermal, and fragmentation effects 4) Explain factors to take into consideration when responding to a potential IED Office for Bombing Prevention IED and Explosive Effects Fundamentals IED Identification Exercise #1 (60 sec) • Identify a partner (teams of 2) • Team member #1 is the “Identifier” • Team member #2 is the “Drawer” • Team member #1 will have a 60 second view of the IED and 5 min to describe the IED to team member #2 for them to draw • Team members will have their backs toward each other • Compare results • Team members switch tasks and repeat • Compare results Office for Bombing Prevention IED and Explosive Effects Fundamentals IED Definition A device placed or fabricated in an improvised manner incorporating destructive, lethal, noxious, pyrotechnic, or incendiary chemicals and designed to destroy, incapacitate, harass, or distract. It may incorporate military stores, but is normally devised from nonmilitary components. Department of Defense Joint Publication 1-02 Dictionary of Military and Associated Terms Office for Bombing Prevention IED and Explosive Effects Fundamentals Why Use IEDs? • Proven effective • Instructions and materials readily available • Inexpensive • Psychological effect Office for Bombing Prevention -

Consumer Fireworks Testing Manual
UNITED STATES OF AMERICA CONSUMER PRODUCT SAFETY COMMISSION CONSUMER FIREWORKS TESTING MANUAL Directorate for Laboratory Sciences Division of Chemistry This manual is to provide guidance to the Commission staff that test fireworks devices for compliance with fireworks regulations. This manual is not intended to supersede or limit fireworks regulations. In the case of discrepancies between this manual and the regulations, the regulations will supersede this manual. This manual has not been reviewed or approved by, and may not necessarily represent the views of, the Commission. Fourth Edition (17 August 2006) TABLE OF CONTENTS I. BACKGROUND.................................................................................................... 4 II. SAFETY AND EQUIPMENT................................................................................ 4 A. Safety Precautions............................................................................................. 4 B. Equipment and Supplies..................................................................................... 5 1. Field Analysis............................................................................................. 5 2. Laboratory Analysis ................................................................................... 5 C. Equipment Calibration and Accuracy................................................................. 6 D. General Fireworks Data and Testing Forms ....................................................... 6 III. SAMPLE ACCOUNTABILITY, HANDLING AND SPLITTING...................... -

Mechanism for Setting a Fuse
(19) & (11) EP 2 390 618 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 30.11.2011 Bulletin 2011/48 F42C 17/00 (2006.01) (21) Application number: 11166830.7 (22) Date of filing: 20.05.2011 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Chiappini, Andrea GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 19136, La Spezia (IT) PL PT RO RS SE SI SK SM TR • Biselli, Gianluca Designated Extension States: 19136, La Spezia (IT) BA ME (74) Representative: Di Gennaro, Sergio (30) Priority: 26.05.2010 IT TO20100439 Barzanò & Zanardo Milano S.p.A. Corso Vittorio Emanuele II, 61 (71) Applicant: Oto Melara S.p.A. 10128 Torino (IT) 19136 La Spezia (IT) (54) Mechanism for setting a fuse (57) A mechanism for setting a fuse, applied to the trol device of the position of the setting device 2, formed structure of a firearm 5, comprising a setting device 2, by a movable equipment 31 from an actuator of a vertical adapted to program such fuse, said setting device 2 being movement 34 and from an actuator of a horizontal move- fixed to a support structure 13 and comprising a reference ment 35. portion 21 and a setting portion 22, rotating around the Such actuators are able to move along a horizontal longitudinal axis of the fuse, adapted to set the fuse of a "X"-axis of the support structure, along a vertical "Z"-axis, cartridge. -

ATF EXPLOSIVES Industry Newsletter June 2013 Published Bi-Annually
U.S. Department of Justice Bureau of Alcohol, Tobacco, Firearms and Explosives ATF EXPLOSIVES Industry Newsletter June 2013 Published Bi-Annually What’s in This Issue New Publication New Publication TF has issued a new pamphlet for firework Exploding Ammunition Requirements manufacturers and persons otherwise involved Smoke Producing Devices in display fireworks. ATF P 5400.24, Fireworks Reagents Reminders, includes information on recordkeeping, tables of distances, marking, transfer and distribution, as well Canadian Type 4 Magazines vs. U.S. Type 2 as recent rulings affecting fireworks storage. The new Magazines publication may be found at http://www.atf.gov/publica- Hardwood or Softwood? tions/explosives-arson.html. This publication is intended as an aid for compliance with statutory and regula- Interior Walls for Type 1 Magazines tory requirements—not as a replacement. The Federal Gun Loading Facilities explosives law at Title 18, United States Code, Chapter 40, provides statutory requirements and implementing Horizontally-Mounted Hoods regulations at 27 CFR, Part 555, provide specific regula- Indoor Storage Reminders tory requirements for explosive materials. Recordkeeping Reminders Permittee Disposal of Surplus Stock Exploding Questions and Answers Ammunition Requirements Explosives Thefts from 2006 thru 2012 TF was recently asked if .50 caliber or smaller Firearms & Explosives Industry Division (FEID) exploding rifle ammunition is exempt as “small Division Chief Debra Satkowiak arms ammunition” under the Federal explosives laws and regulations. Deputy Division Chief Chad J. Yoder In general, firearms ammunition is an “explosive” Explosives Industry Programs Branch (EIPB) because it typically contains smokeless powder and other Branch Chief Paul W. Brown explosive materials. However, 18 U.S.C. -

Chapter 6 INITIATION
Chapter 6 INITIATION COMPONENTS USED IN ELECTRIC BLASTING The electric power source used to initiate a blast may be a twist or push type generator blasting machine and a remote radio device, a condenser discharge machine, or an AC power line through a blasting switch. Never use batteries directly; only a blasting machine or blasting switch provides the current and time control necessary to prevent cap arcing and misfires. The generator type blasting machine converts the mechanical energy of the blaster’s hand motion into electrical energy and then closes the contacts to the firing circuit when the peak electric energy is generated. A condenser discharge machine uses dry-cell batteries to charge a set of capacitors. When the fire button is pushed, the stored electrical energy in these capacitors is discharged into the firing circuit. The remote detona- tion system can be activated and controlled with the commonly used King radio that has been retrofitted with a dual tone modulated frequency chip (DTMF). The entire remote detonation system allows the blaster-in- charge more flexibility in selecting a location to initiate the blast. No alterations or repairs of an electric blasting machine should be attempted, unless by the manufacturer. Ordinary maintenance by the blaster should be limited to changing the batteries in the condenser discharge type machines and to lubricating the moving parts in the generator type. The LEAD WIRE, or the FIRING LINE as it is sometimes called, connects the power source to the blasting circuit. It should be a two-conductor, insulated, solid copper wire (with exception of the multiconduc- tor cable for a sequential blasting machine) and be at least 14-gauge thickness. -
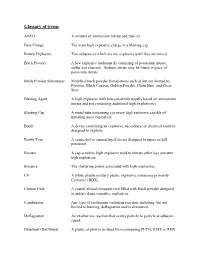
TWGFEX Glossary of Terms
Glossary of terms ANFO A mixture of ammonium nitrate and fuel oil. Base Charge The main high explosive charge in a blasting cap. Binary Explosive Two substances which are not explosive until they are mixed. Black Powder A low explosive traditionally consisting of potassium nitrate, sulfur and charcoal. Sodium nitrate may be found in place of potassium nitrate. Black Powder Substitutes Modified black powder formulations such as but not limited to: Pyrodex, Black Canyon, Golden Powder, Clean Shot, and Clear Shot. Blasting Agent A high explosive with low-sensitivity usually based on ammonium nitrate and not containing additional high explosive(s). Blasting Cap A metal tube containing a primary high explosive capable of initiating most explosives. Bomb A device containing an explosive, incendiary, or chemical material designed to explode. Booby Trap A concealed or camouflaged device designed to injure or kill personnel. Booster A cap sensitive high explosive used to initiate other less sensitive high explosives. Brisance The shattering power associated with high explosives. C4 A white pliable military plastic explosive containing primarily Cyclonite (RDX). Cannon Fuse A coated, thread-wrapped cord filled with black powder designed to initiate flame-sensitive explosives. Combustion Any type of exothermic oxidation reaction, including, but not limited to burning, deflagration and/or detonation. Deflagration An exothermic reaction that occurs particle to particle at subsonic speed. Detasheet (Det Sheet) A plastic explosive in sheet form containing PETN, HMX or RDX. Detonation An exothermic reaction that propagates a shockwave through an explosive at supersonic speed (greater than 3300ft/sec). Detonation Cord (Det-Cord) A plastic/fiber wrapped cord containing a core of PETN or RDX. -

Identification of Artillery Projectiles
IDENTIFICATION OF ARTILLERY. PROJECTILES (WITH ADDENDA) SECOND EDITION RESTRICTED Published by VI Corps June 1944. Republished with Addenda as Second Edition by Seventh^Army Auq 1944 INDEX Preface .......................................................... 2 Sample Shelling Report Form ..........................3 Sec. I - GERMAN................................................ 5 Abbreviations and Nomenclature . 6 Chart: Details of Rotating Bands . 46 Sec. II - ITALIAN.......................................... 51 Chart: Details of Rotating Bands . 57 Sec. I l l - B R IT IS H .......................................6l Chart: Details of Rotating Bands . 65 Sec. IV - AMERICAN.......................................67 Chart: Details of Rotating Bands . 73 Sec. V - MISCELLANEOUS................................75 ADDENDA ............................................................ 79 Inch and Centimeter scale inside back cover. PRgACt. Information included in this booklet haa been compiled from a ll available Intelligence sources, including many original drawings sub mitted by Arty S-2a. No e ffo rt haa been made to indicate the sources o f this information. The measurements on the drawings and in the charts are believed to be reasonably accurate; however, minor varia tions may exist. Binfcmy a rtille ry a ctivity in HALT reached new heights on the ANZ10 Beachhead. Not only have the forward units been shelled continuously, but rear areas including Army, Corps, Air Corps, and Naval installations have been shelled interm ittently with heavy caliber guns. Due to the semi-circular front o f approx 30 miles we have encountered shelling from every direction, and from weapons ranging from the lig h t 75-mm Hecoilless gun to 28-cm super-heavy railway gun. Also, the enemy has used many d iffe re n t types of arty captured from the Russians, Italian s and French. -

Process Safety Management for Explosives and Pyrotechnics Manufacturing
Process Safety Management for Explosives and Pyrotechnics Manufacturing OSHA 3912-03 2017 Occupational Safety and Health Act of 1970 “To assure safe and healthful working conditions for working men and women; by authorizing enforcement of the standards developed under the Act; by assisting and encouraging the States in their efforts to assure safe and healthful working conditions; by providing for research, information, education, and training in the field of occupational safety and health.” This guidance document is not a standard or regulation, and it creates no new legal obligations. It contains recommendations as well as descriptions of mandatory safety and health standards. The recommendations are advisory in nature, informational in content, and are intended to assist employers in providing a safe and healthful workplace. The Occupational Safety and Health Act requires employers to comply with safety and health standards and regulations promulgated by OSHA or by a state with an OSHA‑approved state plan. In addition, the Act’s General Duty Clause, Section 5(a)(1), requires employers to provide their employees with a workplace free from recognized hazards likely to cause death or serious physical harm. Material contained in this publication is in the public domain and may be reproduced, fully or partially, without permission. Source credit is requested but not required. This information will be made available to sensory‑impaired individuals upon request. Voice phone: (202) 693‑1999; teletypewriter (TTY) number: 1‑877‑889‑5627. Process Safety Management for Explosives and Pyrotechnics Manufacturing U.S. Department of Labor Occupational Safety and Health Administration OSHA 3912‑03 2017 Contents Purpose . -

Explosives and Blasting Procedures Manual
Information Circular 8925 Explosives and Blasting Procedures Manual By Richard A. Dick, Larry R. Fletcher, and Dennis V. D'Andrea US Department of Interior Office of Surface Mining Reclamation and Enforcement Kenneth K. Eltschlager Mining/Blasting Engineer 3 Parkway Center Pittsburgh, PA 15220 Phone 412.937.2169 Fax 412.937.3012 [email protected] UNITED STATES DEPARTMENT OF THE INTERIOR James G. Watt, Secretary BUREAU OF MINES Robert C. Horton, Director As the Nation's principal conservation agency, the Department of the Interior has responsibility for most of our nationally owned public lands and natural resources. This includes fostering the wisest use of our land and water re• sources, protecting our fish and wildlife, preserving the environmental and cultural values of our national parks and historical places, and providing for the enjoyment of life through outdoor recreation. The Department assesses our energy and mineral resources and works to assure that their development is in the best interests of all our people. The Department also has a major re· sponsibility for American Indian reservation communities and for people who live in Island Territories under U.S. administration. This publication has been cataloged as follows: Dick, Richard A Explosives and blasting procedures manual, (Bureau of Mines Information circular ; 8925) Supt. of Docs. no.: I 28.27:8925. 1. Blasting-Handbooks, manuals, etc, 2. Explosives-Haodbooks, manuals, etc, I. Fletcher, Larry R. II. D'Andrea, Dennis V. Ill, Title, IV. Series: Information circular (United States, Bureau of Mines) ; 8925, TN295,U4 [TN279] 622s [622'.23] 82·600353 For sale by the Superintendent of Documents, U.S. -

An Initial Study Into Mine Action and Improvised Explosive Devices Geneva International Centre for Humanitarian Demining
AN INITIAL STUDY INTO MINE ACTION AND IMPROVISED EXPLOSIVE DEVICES GENEVA INTERNATIONAL CENTRE FOR HUMANITARIAN DEMINING The GICHD is an expert organisation working to reduce the impact of mines, cluster munitions and other explosive hazards, in close partnership with mine action organisations and other human security organisations. We support the ultimate goal of mine action: saving lives, returning land to productive use and promoting development. Based at the Maison de la paix in Geneva, the GICHD employs around 55 staff members from over 15 different countries. This makes the GICHD a unique and international centre of mine action expertise and knowledge. Our work is made possible by core contributions, project funding and in-kind support from more than 20 governments and organisations. The content of this publication, its presentation and the designations employed do not imply the expression of any opinion whatsoever on the part of the GICHD regarding the legal status of any country, territory or armed groups, or concerning the delimitation of its frontiers or boundaries. All content remains the sole responsibility of the GICHD. Cover: Bob Gravett / MAG An initial study into mine action and improvised explosive devices © GICHD, February 2017 ISBN: 978-2-940369-67-6 AN INITIAL STUDY INTO MINE ACTION AND IMPROVISED EXPLOSIVE DEVICES GENEVA INTERNATIONAL CENTRE FOR HUMANITARIAN DEMINING 2017 1 TABLE OF CONTENTS EXECUTIVE SUMMARY 4 INTRODUCTION 6 IEDs and humanitarian mine action 6 Mine action sector engagement with IEDs 6 The study 6 -

Chapter 11 SPECIALIZED BLASTING TECHNIQUES
Chapter 11 SPECIALIZED BLASTING TECHNIQUES This chapter includes information about: • Avalanche Blasting • Animal Carcass Removal • 105-M 102 Howitzer • Boulder Blasting • Military Weapons • Air Gapping • Fireline Explosives • Expansion Alternatives • Burnol Backfiring Devices SECTION 11A - AVALANCHE BLASTING SECTION CONTENTS: • Initiating Devices • Explosive Assembly • Use of Hand Charge • Cornice Control • Explosive Safety • Recoilless Weapons - Military Weapons • Avalauncher AVALANCHE BLASTING Slope testing, avalanche release, and snow stabilization are the main objectives for using explosives in avalanche blasting. To achieve these objectives, a standard charge is used that is capable of developing deto- nation pressure equal to 1 kg of TNT. 167 There are several types of explosives that can develop the appropriate detonation pressure. By knowing the detonation velocity and the density of a given explosive, the detonation pressure can be calculated (Chapter 2 - Explosives). INITIATING DEVICES Avalanche blasting is based on a nonelectric detonating system or systems that are not susceptible to initiation from the high static electricity that is prevalent in snowstorms and near ridge crests. Even with non- electric blasting caps, avalanche blasting should not be conducted when there is evidence of a strong static electricity field (cumulonimbus clouds, electric buzzing). Cap-and-Fuse - A cap-and-fuse assembly can detonate explosives that are sensitive to a No. 6 cap (Figure 11- 1). However, in severe winter weather, some primers with low proportions of sensitizers may require a No. 8 cap or larger. Blasting caps are susceptible to accidental ignition from excess heat, friction, or static electricity and should be handled with great care. Where adverse conditions are expected (static electricity), other tech- niques should be used or the blasting operation should be shut down.