Signatory Companies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alkermes Public Limited Company
ALKERMES PUBLIC LIMITED COMPANY Directors’ Report and Consolidated Financial Statements For the Year Ended March 31, 2013 ALKERMES PLC Table of Contents Page Directors’ Report .......................................................... 2 Statement of Directors’ Responsibilities .......................................... 56 Independent Auditors’ Report—Group ........................................... 57 Consolidated Profit and Loss Account ........................................... 59 Consolidated Statement of Comprehensive Income (Loss) ............................. 60 Consolidated Balance Sheet ................................................... 61 Consolidated Statement of Cash Flows ........................................... 63 Consolidated Reconciliation of Shareholders’ Funds ................................. 62 Notes to the Consolidated Financial Statements .................................... 64 Independent Auditors’ Report—Company ......................................... 111 Company Balance Sheet ..................................................... 113 Notes to the Company Financial Statements ....................................... 114 1 DIRECTORS’ REPORT For the Year Ended March 31, 2013 The directors present their report and audited consolidated financial statements for the fiscal year ended March 31, 2013. The directors have elected to prepare the consolidated financial statements in accordance with section 1 of the Companies (Miscellaneous Provisions) Act, 2009, which provides that a true and fair view of the state of -

Amgen and Incyte – the Biotechnology Acquisition
Amgen and Incyte – the biotechnology acquisition Ana Carolina Coelho Student number: 152416013 Dissertation written under the supervision of António Luís Borges de Assunção Dissertation submitted in partial fulfilment of requirements for the MSc in Finance, at the Universidade Católica Portuguesa, May 2018 Abstract The main goal of this dissertation is to study the hypothesis of an acquisition in the biotechnology industry, between Amgen (acquirer) and Incyte (target). Amgen is a U.S.-based biotechnology company currently facing a decrease in sales, mainly due to an increase in the market share of generic drugs and the rise of biosimilar products. To offset the poor performance, it is looking for an acquisition in its industry, for which Incyte could be a suitable target. Incyte belongs to the U.S.-biotechnology industry, and although it has a shy presence in the market with only two released drugs, its revenues are expected to increase significantly in the near future. The combination of these companies would allow knowledge transfer about research and development process of new drugs, a stronger position in Europe, and a higher investment power to apply in R&D. The combined company would be able to decrease the number of employees due to duplication of jobs and decrease the cost of sales, as the power over suppliers increases. The potential synergies are valued at $28,522.8 million, of which $16,739.2 million are more likely to be realized. The combined firm, after the introduction of synergies, is expected to generate the same or even higher returns than the sum of the stand- alone businesses and higher growth rates, fulfilling Amgen’s need of growth. -

In Re: Alkermes Securities Litigation 03-CV-12091-Consolidated
Case 1:03-cv-12091-RCL Document 39 Filed 07/12/2004 Page 1 of 49 UNITED STATES DISTRICT COURT DISTRICT OF MASSACHUSETTS In re ALKERMES SECURITIES ) Master Docket No. 03-CV-12091-RCL LITIGATION ) ) CLASS ACTION This Document Relates To: ) ) ALL ACTIONS. ) ) ) CONSOLIDATED COMPLAINT FOR VIOLATION OF THE FEDERAL SECURITIES LAWS Case 1:03-cv-12091-RCL Document 39 Filed 07/12/2004 Page 2 of 49 SUMMARY AND OVERVIEW 1. This is a securities class action on behalf of all purchasers of the common stock of Alkermes, Inc. (“Alkermes” or the “Company”) between April 22, 1999 and July 1, 2002 (the “Class Period”), against Alkermes and certain of its officers and directors for violations of the Securities Exchange Act of 1934 (the “Exchange Act”). 2. Alkermes is a biopharmaceutical company focused on the development of controlled- release drug delivery technologies and their application to existing or new drug therapies. Among the drug delivery technologies defendants seek to develop are sustained-release systems based on biodegradable polymeric microspheres, including those based on Medisorb polymers. 3. In 1996, Alkermes entered into an agreement with JPI Pharmaceutical International (“Janssen”), an affiliate of pharmaceutical giant Johnson & Johnson Pharmaceutical Research & Development, L.L.C. (“Johnson & Johnson”), to develop an injectable form of the schizophrenic drug Risperdal, based upon the Medisorb polymer technology, called Risperdal Consta. Janssen has marketed the oral form of Risperdal since 1993, with sales of $2.3 billion in 2003. According to a December 12, 2001 report by analysts Thomas Weisel Partners, LLC (“Thomas Weisel”), during the Class Period, oral Risperdal was the most prescribed drug in the $5.3 billion atypical antipsychotic market. -

Explanation of Conflict of Interest Disclosure Parts: Part One: All
Explanation of Conflict of Interest Disclosure Parts: Part One: All Financial Involvement with a pharmaceutical or biotechnology company, a company providing clinical assessment, scientific, or medical companies doing business with or proposing to do business with ACNP over past 2 years (Jan. 2011-Present) Part Two: Income Sources & Equity of $10,000 or greater Part Three: Financial Involvement with a pharmaceutical or biotechnology company, a company providing clinical assessment, scientific, or medical products or companies doing business with or proposing to do business with ACNP which constitutes more than 5% of personal income (Jan. 2011-Present): Part Four: Grants from pharmaceutical or biotechnology company, a company providing clinical assessment, scientific, or medical products directly, or indirectly through a foundation, university, or any other organization (Jan. 2011-Present) Part Five: My primary employer is a pharmaceutical/biotech/medical device company. 2012 Program Committee Disclosures Anissa Abi-Dargham: Part 1: Pfizer; Otsuka; Takeda; Sunovion; Shire; Roche; Pierre Favre; Part 2: Pierre Favre William Carlezon: Part 1: Referring to 2010-2011: Scientific Advisory Board, Myneurolab.com; Consultant, Concert Pharmaceuticals; Consultant, Lantheus Medical Imaging; Consultant, Transcept Pharmaceuticals, (Spouse) Senior Scientist, EMD Serono; Part 2: (Spouse) Senior Scientist, EMD Serono, Part 3: (Spouse) Senior Scientist, EMD Serono Cameron Carter: Part1: GlaxoSmithKline research; Part 4: GlaxoSmithKline Karl Deisseroth: Part -

Inozyme Pharma Expands Medical Leadership Team
Inozyme Pharma Expands Medical Leadership Team Strengthens Inozyme’s Ability to Advance Lead Candidate, INZ-701, into Clinical Trials Boston, Mass., Nov. 14, 2019 – Inozyme Pharma Inc., a biotechnology company developing novel medicines to treat rare and life-threatening mineralization disorders, today announced the addition of three industry veterans to its leadership team: • Pedro Huertas M.D., Ph.D., as Chief Medical Officer, • Gus Khursigara Ph.D., as Vice President of Medical Affairs and Clinical Operations, and • Catherine Nester, as Vice President of Physician and Patient Strategies. The executives will be instrumental in advancing Inozyme’s lead drug candidate, INZ-701, into clinical trials in 2020 for the treatment of patients with ENPP1 deficiency. INZ-701 is the first therapy that addresses the pathology of ENPP1 deficiency, including diseases such as generalized arterial calcification of infancy (GACI) type 1 and autosomal recessive hypophosphatemic rickets type 2 (ARHR2), both of which are rare and life-threatening manifestations of this enzyme deficiency. Inozyme Pharma received orphan drug designation for INZ-701 in the US and EU in 2018. “We are pleased and excited to welcome Pedro, Gus and Catherine to the Inozyme team,” said Axel Bolte, co-founder and chief executive officer of Inozyme. “Their expertise in orphan drug development – spanning medical affairs, regulatory affairs and clinical development – will strengthen and accelerate our ability to bring potentially life-saving medicines to people who urgently need effective treatments.” Dr. Huertas, a veteran of the pharmaceutical industry, has extensive experience in research and development and medical and regulatory affairs, especially regarding rare disorders and enzyme replacement therapies. -

ALEXION PHARMACEUTICALS, INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K Current Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event reported): May 4, 2021 ALEXION PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) Delaware 000-27756 13-3648318 (State or other jurisdiction of incorporation) (Commission File Number) (IRS Employer Identification No.) 121 Seaport Boulevard, Boston, Massachusetts 02210 (Address of principal executive offices, including zip code) (475) 230-2596 (Registrant’s telephone number, including area code) Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions: ☒ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) ☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) ☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) ☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Securities registered pursuant to Section 12(b) of the Act: Trading Name of each exchange Title of each class Symbol on which registered Common Stock, par value $0.0001 per share ALXN The Nasdaq Global Select Market Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). -

1976 3M Medical Solutions Division 2019 Electrocore, Inc. 2019 Optinose 1985 Abbott Laboratories, Inc
CURRENT SUSTAINING MEMBER COMPANIES MEMBER FOR OVER: 10 Years 25 Years 50 Years Member Since (alphabetical order) 1976 3M Medical Solutions Division 2019 electroCore, Inc. 2019 Optinose 1985 Abbott Laboratories, Inc. 2010 Endo Pharmaceuticals 2018 Organogenesis 2013 AbbVie Inc. 2017 Exelixis 2004 Otsuka America Pharmaceutical, Inc. 2021 Adaptive Biotechnologies 2016 Express Scripts Federal Pharmacy 2018 Pacira BioSciences, Inc. 2017 ACADIA Pharmaceuticals, Inc. 2010 Federal Practitioner 2018 Paratek Pharmaceuticals 2020 AcelRx Pharmaceuticals, Inc. 2018 Foundation Medicine, Inc. 1990 Pfizer Pharmaceuticals 2020 Acorda Therapeutics 2021 Frontier Technology Inc. (FTI) 2017 Pharmacyclics, LLC 2019 Aimmune 2020 Fresenius Medical Care North America 2020 RedHill BioPharma 2003 Alcon Laboratories, Inc. 1989 Genentech Inc. 2019 Red One Medical 2019 Alexion Pharmaceuticals, Inc. 2006 Gilead Sciences 2020 Regeneron 2017 Alkermes, Inc. 1983 GLAXOSMITHKLINE 2009 Regenesis Biomedical, Inc. 2019 Alnylam Pharmaceuticals 2013 Golden State Medical Supply, Inc. 2011 Remund Group, LLC 2019 Altarum Institute 2020 GRAIL 2018 Rigel Pharmaceuticals 2020 Amarin Corporation 2019 Greenwich Biosciences 2000 Sanofi 1994 AmerisourceBergen 2013 Gulf Coast Pharmaceuticals Plus, LLC 2020 Seattle Genetics 1992 Amgen 2008 Heritage Health Solutions, Inc. 2004 Siemens Medical Solutions 2020 Amneal Pharmaceutical 2017 Hill-Rom Company 2019 SK Life Science, Inc. 2019 Aptive Resources LLC 2020 Immunomedics 2002 Smith & Nephew, Inc. 2020 The Arbinger Institute 2019 ImmunoVation, LLC 2019 Sobi Inc. 2011 Arbor Pharmaceuticals, LLC 2019 Incyte Corporation 2013 Stryker Orthopaedics 2010 Argentum Medical, LLC 2019 Indivior 2018 Sun Pharmaceutical 2019 ASM Research, LLC 2015 Intercept Pharmaceuticals 1999 Sunovion Pharmaceuticals, Inc. 1986 Astellas Pharma US, Inc. 2019 Ipsen Biopharmaceuticals, Inc. 2016 Taiho Oncology, Inc. 1995 AstraZeneca 2018 IT Cadre 2015 Takeda Oncology 2020 Baudax Bio, Inc. -
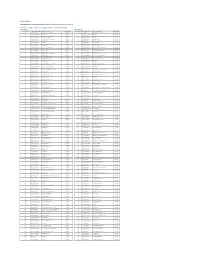
Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN)
Date: 20-Aug-21 Index Weights as of monthly rebalance date 10-Aug-21 Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN) Long Exposure Short Exposure Constituent Bloomberg Ticker Constituent Name Weight(%) Constituent Bloomberg Ticker Constituent Name Weight(%) 1 AAP UN Equity Advance Auto Parts Inc 0.24% 1 A UN Equity Agilent Technologies Inc -0.12% 2 ABBV UN Equity AbbVie Inc. 0.59% 2 HWM UN Equity Alcoa Inc -1.02% 3 ABC UN Equity AmerisourceBergen Corp 0.06% 3 AAL UW Equity American Airlines Group Inc -1.09% 4 ADBE UW Equity Adobe Systems Inc 0.01% 4 AAPL UW Equity Apple Inc. -0.46% 5 ADM UN Equity Archer-Daniels-Midland Co 0.26% 5 ABMD UW Equity ABIOMED Inc -0.11% 6 ADSK UW Equity Autodesk Inc 0.26% 6 ABT UN Equity Abbott Laboratories -0.26% 7 AES UN Equity AES Corp 0.37% 7 CB UN Equity ACE Limited -0.07% 8 AIG UN Equity American Intl Group Inc 0.52% 8 ACN UN Equity Accenture plc -0.29% 9 AIZ UN Equity Assurant Inc 0.11% 9 ADI UW Equity Analog Devices Inc -0.13% 10 ALGN UW Equity Align Technology Inc 0.59% 10 ADP UW Equity Automatic Data Processing -0.76% 11 ALL UN Equity Allstate Corp 0.16% 11 AEE UN Equity Ameren Corp -0.24% 12 ALLE UN Equity Allegion PLC 0.34% 12 AEP UW Equity American Electric Power -0.23% 13 AMAT UW Equity Applied Materials Inc 0.59% 13 AFL UN Equity AFLAC Inc -0.29% 14 AMD UW Equity Advanced Micro Devices Inc 1.15% 14 AJG UN Equity ARTHUR J GALLAGHER & CO -0.23% 15 AME UN Equity AMETEK Inc 0.26% 15 AKAM UW Equity Akamai Technologies Inc -0.11% 16 AMT UN Equity American Tower Corp A 0.39% 16 ALB UN -

Corporate Responsibility Report
Corporate Responsibility Report October 2019 ©2019 Alkermes. All rights reserved. Table of Contents Section 1: Introduction . 2 Section 2: About Our Company . 3 Section 3: Our Approach to Corporate Responsibility . 4 Section 4: Environment . 6 Section 5: Social . 15 Section 6: Governance . 23 Section 7: The Future of Corporate Responsibility at Alkermes and About This Report . 27 ALKERMES • CORPORATE RESPONSIBILITY REPORT 1 SECTION 1 INTRODUCTION A Message from Our CEO Alkermes was founded by pioneers in the field of Inspired by the courage and determination of individuals neuroscience, and their legacy of innovation and in these frequently underserved and marginalized scientific excellence — applied to the real-world needs communities, we are driven to develop medicines and of patients — remains central to our mission. Alkermes contribute to systemic solutions that we hope will have technologies and discoveries have contributed to a meaningful impact on the lives of patients. important medicines that continue to shape the treatment landscape in many disease areas. Today, Our Commitment we are distinguished from other biopharmaceutical As we look toward building our business for the companies by our core focus on serious mental illness future, we are guided by the opportunity — and what and addiction — chronic, highly prevalent conditions we believe is our responsibility — to help address the that affect millions of people and represent some of unmet needs of patients and to operate in a sustainable, the most challenging public health issues of our time. socially responsible manner. We firmly believe that doing so is best for our business, our employees, for Great science. Deep compassion. -

Incyte Corporation 2016 Annual Report
2016 ANNUAL REPORT TABLE OF CONTENTS Letter to Shareholders 2 Key Planned Goals for 2017 6 Innovation 7 Growth 10 Strength 13 Corporate Responsibility 15 Company Information 19 ii Incyte’s Executive Management Team LETTER TO SHAREHOLDERS Standing, left to right: Steven H. Stein, MD; Dear Shareholders, Paula J. Swain; Wenqing Yao, PhD; Jonathan E. Dickinson; At Incyte, we believe that innovation and the discovery of new products David W. Gryska; creates long-term value for patients and society, as well as for our employ- Hervé Hoppenot; Michael Morrissey; ees and our shareholders. It is our commitment to these objectives that has Vijay Iyengar, MD enabled us to make significant progress in the last year. During 2016, we saw continued growth in the number of patients being treated with Jakafi® Seated, left to right: Eric H. Siegel, JD, MBA; (ruxolitinib), our JAK1/JAK2 inhibitor, and we also added Iclusig® (ponati- Reid M. Huber, PhD; nib) to our commercial portfolio as part of our European transaction with Barry P. Flannelly, PharmD, MBA ARIAD Pharmaceuticals, Inc. In February 2017, with Eli Lilly & Company, we announced the European approval of Olumiant® (baricitinib). I believe that we are on Summary of Revenue-Generating Products track to reach our goal of PRODUCT NAME DRUG NAME TARGET APPROVED TERRITORY INDICATION(S) becoming a world-class, Jakafi® ruxolitinib1 JAK1/JAK2 Global MF3; PV4 Jakavi® global biopharmaceutical organization. For the first Iclusig® ponatinib BCR-ABL Europe CML and Ph+ ALL5 time in the history of our company, Incyte’s total Olumiant® baricitinib2 JAK1/JAK2 Europe RA6 yearly revenue surpassed $1 billion in 2016. -

Massmutual Mid Cap Growth Fund T
Fund Holdings As of 06/30/2021 MassMutual Mid Cap Growth Fund T. Rowe Price | Frontier Capital Prior to 5/1/2021, the Fund name was MassMutual Select Mid Cap Growth Fund. Fund Shares or Par Position Market Security Name Ticker CUSIP Weighting (%) Amount Value ($) Microchip Technology Inc MCHP 595017104 2.07 1,348,381 201,906,571 Agilent Technologies Inc A 00846U101 1.90 1,252,717 185,164,100 Hologic Inc HOLX 436440101 1.84 2,678,942 178,739,010 Teleflex Inc TFX 879369106 1.82 441,297 177,308,722 Ball Corp BLL 058498106 1.75 2,104,895 170,538,593 Textron Inc TXT 883203101 1.55 2,199,000 151,225,230 Burlington Stores Inc BURL 122017106 1.54 465,929 150,024,479 Catalent Inc CTLT 148806102 1.53 1,376,000 148,773,120 Marvell Technology Inc MRVL 573874104 1.46 2,442,368 142,463,325 Bruker Corp BRKR 116794108 1.39 1,776,000 134,940,480 Ingersoll Rand Inc IR 45687V106 1.29 2,564,000 125,148,840 The Cooper Companies Inc COO 216648402 1.28 314,645 124,684,374 KKR & Co Inc Ordinary Shares KKR 48251W104 1.25 2,050,813 121,490,162 Caesars Entertainment Inc CZR 12769G100 1.13 1,057,315 109,696,431 Reserve Invt Fds 0 76105Y208 1.07 103,877,547 103,877,547 DocuSign Inc DOCU 256163106 1.05 366,000 102,322,620 Chipotle Mexican Grill Inc CMG 169656105 1.04 65,283 101,210,846 Dollar General Corp DG 256677105 1.02 460,009 99,541,348 Veeva Systems Inc Class A VEEV 922475108 1.00 314,189 97,697,070 Avantor Inc AVTR 05352A100 0.98 2,700,000 95,877,000 JB Hunt Transport Services Inc JBHT 445658107 0.98 585,000 95,325,750 KLA Corp KLAC 482480100 0.97 291,710 94,575,299 -

Pharma: Strategic Realignment for a 112/113 Better Future Prism / 2 / 2020
Pharma: Strategic realignment for a 112/113 better future Prism / 2 / 2020 Pharma: Strategic realignment for a better future ... and how the industry will be forced to overcome its hesitation to innovate in operations Ben van der Schaaf, Aurelien Guichard The life sciences sector faces significant impact from Amid the search for COVID-19 – and although the race for treatments and vaccines effective COVID-19 treatments and vaccines, is dominating the headlines, the effect on the industry will the pandemic will have not solely be positive. long-term side effects for the global pharmaceutical In the short term, some companies industry. As our article explains, companies are laying people off and reducing will need to focus on operations, whereas others are change in three areas reallocating resources to focus on (portfolio reprioritization, COVID-19, or even ramping up accelerated R&D and technology efforts in other areas. Stock-market transformation) if they performance has been as diverse are to position (Figure 1). themselves successfully for the future. Organizations need to consider major strategic questions now, to ensure their success in the longer term. The industry is clearly in the middle of the efforts to combat COVID-19: - More than 20 companies are trying to find a treatment with either new or approved drugs1. - More than 15 companies globally are mobilizing resources to develop new vaccines2. - Globally, by the end of May, more than 1,300 clinical trials related to COVID-19 were recruiting patients3. 1. Marketwatch.com, 6 May 2020 2. Drugtargetreview.com, 9 April 2020 3. clintrials.gov, 31 May 2020 Pharma: Strategic realignment for a 114/115 better future Prism / 2 / 2020 2.