Pilot Plant Study to Utilize Waste Brine Generated by Salt Industries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
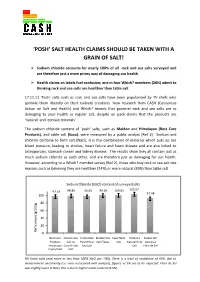
Latest Press Release from Consensus Action on Salt and Health
‘POSH’ SALT HEALTH CLAIMS SHOULD BE TAKEN WITH A GRAIN OF SALT! Sodium chloride accounts for nearly 100% of all rock and sea salts surveyed and are therefore just a more pricey way of damaging our health Health claims on labels fuel confusion; one in four Which? members (28%) admit to thinking rock and sea salts are healthier than table salt 17.11.11 ‘Posh’ salts such as rock and sea salts have been popularised by TV chefs who sprinkle them liberally on their culinary creations. New research from CASH (Consensus Action on Salt and Health) and Which? reveals that gourmet rock and sea salts are as damaging to your health as regular salt, despite on pack claims that the products are ‘natural’ and ‘contain minerals’. The sodium chloride content of ‘posh’ salts, such as Maldon and Himalayan (Best Care Products), and table salt (Saxa), were measured by a public analyst [Ref 1]. Sodium and chloride combine to form salt (NaCl), it is this combination of minerals which puts up our blood pressure, leading to strokes, heart failure and heart disease and are also linked to osteoporosis, stomach cancer and kidney disease. The results show they all contain just as much sodium chloride as each other, and are therefore just as damaging for our health. However, according to a Which? member survey [Ref 2], those who buy rock or sea salt cite reasons such as believing they are healthier (24%) or more natural (39%) than table salt. Sodium Chloride (NaCl) content of surveyed salts 98.86 96.65 99.50 100.65 103.57 97.19 91.48 100 80 60 40 20 NaCl contentNaCl (g/100g) 0 Best Care Cornish Sea Halen Mon Maldon Sea Saxa Table Tidman's Zauber der Products Salt Co Pure White Salt Flakes Salt Natural Rock Gewürze Himalayan Cornish Sea Sea Salt Salt Fleur de Sel Crystal Salt Salt NB Some salts total more or less than 100% NaCl per 100g. -

OCCASION This Publication Has Been Made Available to the Public on The
OCCASION This publication has been made available to the public on the occasion of the 50th anniversary of the United Nations Industrial Development Organisation. DISCLAIMER This document has been produced without formal United Nations editing. The designations employed and the presentation of the material in this document do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations Industrial Development Organization (UNIDO) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries, or its economic system or degree of development. Designations such as “developed”, “industrialized” and “developing” are intended for statistical convenience and do not necessarily express a judgment about the stage reached by a particular country or area in the development process. Mention of firm names or commercial products does not constitute an endorsement by UNIDO. FAIR USE POLICY Any part of this publication may be quoted and referenced for educational and research purposes without additional permission from UNIDO. However, those who make use of quoting and referencing this publication are requested to follow the Fair Use Policy of giving due credit to UNIDO. CONTACT Please contact [email protected] for further information concerning UNIDO publications. For more information about UNIDO, please visit us at www.unido.org UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Tel: (+43-1) 26026-0 · www.unido.org · [email protected] MK'Jif iO ipv P[ )| I j Г ¡r irj IP - -T r,HAH î IZSég i Restricted bwirw^l. -

The Changing Technology of Post Medieval Sea Salt Production in England
1 Heritage, Uses and Representations of the Sea. Centro de Investigação Transdisiplinar Cultura, Espaço e Memoría (CITCEM) Porto, Faculdade de Letras da Universidade do Porto, 20-22 October 2011. The changing technology of post medieval sea salt production in England Jeremy Greenwood Composition of seawater Sea water contains 3.5% evaporites of which salt (sodium chloride) comprises 77.8%. The remainder is known as bittern as it includes the bitter tasting, aperient and deliquescent sulphates of magnesium (Epsom salt) and sodium (Glauber’s salt) as well as about 11% magnesium chloride. 2 Successful commercial salt making depends on the fractional crystallisation of seawater producing the maximum amount of salt without contamination by bittern salts. As seawater is evaporated, very small amounts of calcium carbonate are precipitated followed by some calcium sulphate. This is followed by the crystallisation of sodium chloride but before this is complete, bitter Epsom salt appears; something that needs to be avoided.1 In Continental Europe, evaporation of sea water is achieved solely by the energy of the wind and sun but this is not possible in the English climate so other techniques were developed. 1 http://www.solarsaltharvesters.com/notes.htm SOLAR SALT ENGINEERING 3 Evaporation vessel Briquetage The earliest known English method of coastal saltmaking has been found in the late Bronze Age. This involved boiling seawater in crude clay dishes supported by clay firebars (briquetage) and was widespread in Europe. This technique continued into the Iron Age and into the Roman period with variations inevitably occurring in the industry, although the dating of saltworks is very problematical.2 Detailed interpretation continues to be a matter of dispute. -

LAS VEGAS PRODUCT CATALOG INGREDIENTS Full Page Ad for FINE PASTRY 11”X 8.5”
PRODUCT CATALOG LAS VEGAS chefswarehouse.com BAKING AND PASTRY FROZEN/RTB BREAD ...................12 BEVERAGES, GOAT CHEESE ............................21 CONDIMENTS BAKING JAM ..............................4 PIZZA SHELLS ...............................12 COFFEE AND TEA GOUDA.......................................21 AND JAMS TORTILLAS/WRAPS ......................12 HAVARTI.......................................22 BAKING MIXES ............................4 BAR MIXERS ................................17 CHUTNEY ....................................25 WRAPPERS ..................................12 JACK CHEESE .............................22 BAKING SUPPLIES .......................4 BITTERS .........................................17 GLAZES AND DEMI-GLAZES .......25 BROWNIES ..................................12 MASCARPONE ...........................22 COLORANTS ...............................4 CORDIAL ....................................17 KETCHUP .....................................25 CAKES ASSORTED ......................12 MISCELLANEOUS ........................22 CROISSANTS ...............................4 JUICE ...........................................17 MAYO ..........................................25 TARTS ...........................................13 MOUNTAIN STYLE ........................22 DÉCOR ........................................4 MISCELLANEOUS ........................17 MUSTARD ....................................25 COULIS ........................................13 MOZZARELLA ..............................22 EXTRACTS ....................................6 -

Report on the Composition of Prevalent Salt Varieties
Federal Department of Home Affairs FDHA Federal Food Safety and Veterinary Office FSVO Nutrition Esther Infanger, Max Haldimann, Mai 2016 Report on the composition of prevalent salt varieties 071.1/2013/16500 \ COO.2101.102.7.405668 \ 000.00.61 Contents Summary ................................................................................................................................................. 3 Zusammenfassung .................................................................................................................................. 4 Synthèse .................................................................................................................................................. 5 Sintesi .................................................................................................................................................... 6 1 Introduction ................................................................................................................................. 7 2 Starting point............................................................................................................................... 7 3 Methods ...................................................................................................................................... 8 4 Results ........................................................................................................................................ 9 5 Discussion ............................................................................................................................... -

Epicurean Product Guide 2016 V6.Xlsx
Epicurean Product Listing 2016 800.934.6495 173 Thorn Hill Rd Warrendale, PA 15086 ** For the most up to date listing, please visit our website ** version 6, 9/27/16 EPICUREAN PRODUCT LISTING Condiments.........................................3 Miscellaneous......................................8 Oils & Vinegars................................1210 Syrups.............................................1513 Spices.............................................1715 Dried Mushrooms............................2321 Dried Fruits & Nuts..........................2422 Breads and Crackers.......................2724 Meats & Seafood.............................3027 Pasta Sauces and Noodles.............3330 Desserts..........................................3633 Chocolate........................................4037 Grains & Legumes...........................4340 Cheese, Dairy, & Eggs....................4542 Bar & Bakery.......................................47 Baking & Pastry...................................50 Appetizers...........................................61 CONDIMENTS Prod # Description Packaging UoM Special Order 06206 BASE BEEF NO MSG 4/5 LB CS 06207 BASE BEEF NO MSG 5 LB EA 06176 BASE BEEF NO MSG MINORS 12/1 LB CS X 06179 BASE CHICKEN NO MSG 1 LB EA 06201 BASE CHICKEN NO MSG 5 LB EA 06178 BASE CHICKEN NO MSG MINORS 12/1 LB CS 06200 BASE CHICKEN NO MSG MINORS 4/5 LB CS 06180 BASE CLAM NO MSG MINORS 6/1 LB CS 06181 BASE CLAM NO MSG MINORS 1# EA 06198 BASE CRAB NO MSG MINORS 6/1 LB CS 06199 BASE CRAB NO MSG MINORS 1# EA 06187 BASE ESPAGNOLE SAUCE -

Solar Salt Technology in Ghana – a Case Study of Small Scale Salt Winning Process
Ghana J. Sci. 46 (2006), 99-109 SOLAR SALT TECHNOLOGY IN GHANA – A CASE STUDY OF SMALL SCALE SALT WINNING PROCESS B. MENSAH AND R. BAYITSE CSIR – Institute of Industrial Research, P. O. Box M.32, Accra, Ghana Abstract Résumé Generally, unrefined salt from local small scale salt MENSAH B. & BAYITSE R.: Technologie solaire de sel au producers does not meet the standard specifications Ghana - Une étude de cas du procé dé de salinage of the Ghana Standard Board. In this study, the peu important. En général le sel non raffiné de saliniers traditional small scale salt production process was locaux peu importants ne correspond pas aux studied. Brine and salt samples from the various spécifications normales du Conseil Ghanéen de stages of the traditional process were analysed for Normalisation. Dans cette étude le procédé calcium, sulphate, magnesium and chloride contents traditionnel relatid à la production de sel peu impor- in order to propose a method to improve the quality tant, était étudié. L’eau salée et les échantillons de sel of output. The brine samples contained low levels of prélevés de différents stades du procédé traditionnel calcium (ranging from 0.14% to 0.01% ), and high étaient analysés pour les teneurs en calcium, en sul- levels of magnesium (ranging from 1.04% to 4.49%). fate, en magnésium et en chlorure afin de proposer These two elements constitute the two main une méthode pour améliorer la qualité de la produc- elemental impurities found in common salt. Samples tion. Les échantillons contenaient de faibles niveaux of salt produced also contain high levels of de calcium et de niveaux élevés de magnésium, les magnesium, averaging 0.98 per cent, above the deux impuretés élémentaires principales qui se trouvent recommended maximum level of 0.1 per cent by the dans le sel ordinaire. -

Saltworks by Jennifer Stone Gaines and John York
From Spritsail: A Journal of the History of Falmouth and Vicinity, Vol. 21, No. 1. Winter, 2007. Woods Hole Historical Collection, Woods Hole, MA Saltworks by Jennifer Stone Gaines and John York In Colonial days, a large quantity of salt was abso England fisheries until the end of the 1600s when lutely essential to life in New England, both for food the development of Mediterranean style salt works preservation and for income. One of the best ways in the British Caribbean islands made high quality to preserve fish and meat was to salt it. To preserve salt available to all the British colonies. Salt became cod, the fish were gutted, beheaded, and put into a common return cargo for vessels carrying New wooden barrels, layered with salt - almost as much England exports, primarily timber and salt fish, to salt as fish. The fish sat in the barrels for a week to the Caribbean colonies. ten days while the salt pulled moisture out of the fish. The barrels were then ope:nea , tne orine aumpea out, This arrangement worked well until the Ameri and then the fish put ba ck into the barrels, layered can colonies began with fresh salt. After to chafe under Brit several more days, the ish restrictions. The brine was dumped British government out and the fish were imposed not only the spread on racks to infamous tax on tea, dry in the sun. When but also a salt tax. thoroughly dry, the Since salt was criti fish were put into cal to the livelihood clean dry barrels, of the Massachusetts ready for export. -

Precipitation of Nano-Magnesium Hydroxide from Bittern Using Ultrasound Irradiation
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Der Chemica Sinica, 2013, 4(2):69-81 ISSN: 0976-8505 CODEN (USA) CSHIA5 Precipitation of nano-magnesium hydroxide from bittern using ultrasound irradiation Sh. El Rafie and M. S. Mohamed National Research Center, Chemical Engineering Department, Cairo, Egypt _____________________________________________________________________________________________ ABSTRACT Fiber and platelet-like Nano-sized Mg (OH) 2 particles were synthesized by precipitation of Magnesium rich Bitten 2+ 73 g/L Mg and ammonia water as precipitator. The influence of aging process on Mg (OH) 2 suspension was investigated. The aid of ultrasonic irradiation was studied via synthesis of Mg (OH) 2 one-step precipitation reaction. The morphology and properties of the product was analyzed by X-ray diffraction, scanning electron microscope SEM, Fourier transform infrared spectroscopy FT-IR. Thermal analysis TGA –TDA assisted the high decomposition of Mg(OH) 2 which can be used as green flame retardant filler. The results showed Mg(OH) 2 particles with fiber-like morphology and platelet morphology were obtained with ammonium as precipitator and product was intensively modified by aging process and/or ultrasound waves which increased the surface area and decreased the particle size of Mg(OH) 2. Moreover Ultrasound irradiation demonstrated to be a simple and fast method to assist preparation of high crystalline Mg(OH) 2 .The Nano-crystals size was verified with Transmittance electron microscope TEM. The -

Seawater Bittern a Precursor for Magnesium Chloride Separation
l o rna f Wa ou s J te l a R n e Abdel-AAl et al., Int J Waste Resour 2017, 7:1 o s i o t u International Journal a r n c r DOI: 10.4172/2252-5211.1000267 e e t s n I ISSN: 2252-5211 of Waste Resources ResearchReview Article Article Open Access Seawater Bittern a Precursor for Magnesium Chloride Separation: Discussion and Assessment of Case Studies Hussein Abdel-Aal1*, Khaled Zohdy2 and Maha Abdelkreem2 1Emeritus of Chemical Engineering, NRC, Cairo, Egypt 2Department of Chemical Engineering, Higher Technological Institute, Tenth of Ramadan City, Egypt Abstract Sea water bitterns (SWB) are encountered in the processes of desalination and sea-salt production where large quantities of bitterns and brines are produced, either as by-products, or as waste products. They could be described as “exhausted brines”. In theory, for every ton of sea-salt produced about one cubic meter of bittern is produced and is available for further processing. To exploit valuable salt products, in particular MgCl2 from sea water in desalination plants and/or salt production, various methods were carried out, in particular by the author and his colleagues. Mainly they consist in applying the physical concept of preferential-type of salt separation, where Mg Cl2 is the most soluble salt, will separate at the very end. An experimental work was initiated by Kettani and Abdel-Aal and extended by Abdel-Aal et al. Two case studies are presented and discussed in this paper. Keywords: Desalination; Exhausted brines; Magnesium chloride out, yielding about 75% of the available sodium chloride (known as halide) in solution. -

PHOENIX Chefswarehouse.Com BAKING and PASTRY TORTILLAS/WRAPS
PRODUCT CATALOG PHOENIX chefswarehouse.com BAKING AND PASTRY TORTILLAS/WRAPS......................8 BEVERAGES, HAVARTI.......................................19 CONDIMENTS BAKING MIXES ............................4 WRAPPERS..................................8 COFFEE AND TEA JACK CHEESE .............................19 AND JAMS BROWNIES ..................................10 MASCARPONE ...........................19 BAKING SUPPLIES .......................4 BAR MIXERS ................................15 CHUTNEY ....................................24 CAKES ASSORTED ......................10 MISCELLANEOUS........................19 COLORANTS ...............................4 CORDIAL ....................................15 GLAZES AND DEMI-GLAZES .......24 TARTS ...........................................10 MOUNTAIN STYLE........................19 CROISSANTS ...............................4 JUICE ...........................................15 KETCHUP .....................................24 COULIS........................................10 MOZZARELLA..............................20 DÉCOR ........................................4 MISCELLANEOUS BEVERAGE ....15 MAYO ..........................................24 PUREE..........................................10 MUENSTER...................................20 EXTRACTS ....................................4 NECTAR .......................................15 MUSTARD ....................................24 ZEST..............................................10 PARMESAN .................................20 FILLING ........................................4 -

Report on the Composition of Prevalent Salt Varieties
Federal Department of Home Affairs FDHA Federal Food Safety and Veterinary Office FSVO Nutrition Esther Infanger, Max Haldimann, Mai 2016 Report on the composition of prevalent salt varieties 071.1/2013/16500 \ COO.2101.102.7.405668 \ 000.00.61 Contents Summary ................................................................................................................................................. 3 Zusammenfassung .................................................................................................................................. 4 Synthèse .................................................................................................................................................. 5 Sintesi .................................................................................................................................................... 6 1 Introduction ................................................................................................................................. 7 2 Starting point............................................................................................................................... 7 3 Methods ...................................................................................................................................... 8 4 Results ........................................................................................................................................ 9 5 Discussion ...............................................................................................................................