Meteoric Be and Be As Process Tracers in the Environment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Radiochemistry of Beryllium
National Academy of Sciences National Research Council I NUCLEAR SCIENCE SERIES The Radiochemistry ·of Beryllium COMMITTEE ON NUCLEAR SCIENCE L. F. CURTISS, Chairman ROBLEY D. EVANS, Vice Chairman National Bureau of Standards MassaChusetts Institute of Technol0gy J. A. DeJUREN, Secretary ./Westinghouse Electric Corporation H.J. CURTIS G. G. MANOV Brookhaven National' LaboratOry Tracerlab, Inc. SAMUEL EPSTEIN W. WAYNE MEINKE CalUornia Institute of Technology University of Michigan HERBERT GOLDSTEIN A.H. SNELL Nuclear Development Corporation of , oak Ridge National Laboratory America E. A. UEHLING H.J. GOMBERG University of Washington University of Michigan D. M. VAN PATTER E.D.KLEMA Bartol Research Foundation Northwestern University ROBERT L. PLATZMAN Argonne National Laboratory LIAISON MEMBERS PAUL C .. AEBERSOLD W.D.URRY Atomic Energy Commission U. S. Air Force J. HOW ARD McMILLEN WILLIAM E. WRIGHT National Science Foundation Office of Naval Research SUBCOMMITTEE ON RADIOCHEMISTRY W. WAYNE MEINKE, Chairman HAROLD KIRBY University of Michigan Mound Laboratory GREGORY R. CHOPPIN GEORGE LEDDICOTTE Florida State University. Oak Ridge National Laboratory GEORGE A. COW AN JULIAN NIELSEN Los Alamos Scientific Laboratory Hanford Laboratories ARTHUR W. FAIRHALL ELLIS P. STEINBERG University of Washington Argonne National Laboratory JEROME HUDIS PETER C. STEVENSON Brookhaven National Laboratory University of California (Livermore) EARL HYDE LEO YAFFE University of CalUornia (Berkeley) McGill University CONSULTANTS NATHAN BALLOU WILLIAM MARLOW Naval Radiological Defense Laboratory N atlonal Bureau of Standards JAMESDeVOE University of Michigan CHF.MISTRY-RADIATION AND RADK>CHEMIST The Radiochemistry of Beryllium By A. W. FAIRHALL. Department of Chemistry University of Washington Seattle, Washington May 1960 ' Subcommittee on Radiochemistry National Academy of Sciences - National Research Council Printed in USA. -

EXTRACTION of BERYLLIUM-10 from SOIL by FUSION Summary
EXTRACTION OF BERYLLIUM-10 FROM SOIL BY FUSION Summary This method is used to separate Be from soil and sediment samples, for AMS analysis. After adding Be carrier, the sample is fused with KHF2. Be is extracted from the fusion cake with hot water. K is removed by precipitation, the sample is dried to expel fluoride, and Be is recovered as Be(OH)2. Yields are typically 70-80 %, but can be increased by additional leaching of the fusion cake. Version Written by John Stone, 1996-2002. This version revised May 2004 by Greg Balco. Available at: http://depts.washington.edu/cosmolab/chem.html References If you use this method, please cite: Stone, J.O.H. (1998). A rapid fusion method for the extraction of Be-10 from soils and silicates. Geochimica et Cosmochimica Acta (Scientific comment) 62, 555-561. Sampling and grinding Mix and grind an unbiased ∼ 50-100 g sample of the material. If possible, mix the original sample bag before opening and take representative proportions of coarse and fine material. Fine soil, carbonate nodules, clay lumps, rock fragments, etc., all have different 10Be concentrations. Grinding homogenises the sample and ensures that these components are evenly represented in the small subsample used for the analysis. If the sample is wet, you will need to dry it before grinding. Initial sample drying Samples are best run in batches of four. This is because we currently have four Pt crucibles. Given more crucibles and sufficient hotplate space, batches of 8 or even 12 are equally feasible. ————————————————— UW Cosmogenic Nuclide Lab – 10Be fusion process 1 – Label and tare four disposable aluminum foil drying dishes and transfer a few grams of ground sediment to each with a steel spatula. -
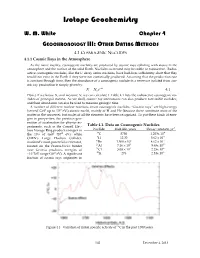
Isotopegeochemistry Chapter4
Isotope Geochemistry W. M. White Chapter 4 GEOCHRONOLOGY III: OTHER DATING METHODS 4.1 COSMOGENIC NUCLIDES 4.1.1 Cosmic Rays in the Atmosphere As the name implies, cosmogenic nuclides are produced by cosmic rays colliding with atoms in the atmosphere and the surface of the solid Earth. Nuclides so created may be stable or radioactive. Radio- active cosmogenic nuclides, like the U decay series nuclides, have half-lives sufficiently short that they would not exist in the Earth if they were not continually produced. Assuming that the production rate is constant through time, then the abundance of a cosmogenic nuclide in a reservoir isolated from cos- mic ray production is simply given by: −λt N = N0e 4.1 Hence if we know N0 and measure N, we can calculate t. Table 4.1 lists the radioactive cosmogenic nu- clides of principal interest. As we shall, cosmic ray interactions can also produce rare stable nuclides, and their abundance can also be used to measure geologic time. A number of different nuclear reactions create cosmogenic nuclides. “Cosmic rays” are high-energy (several GeV up to 1019 eV!) atomic nuclei, mainly of H and He (because these constitute most of the matter in the universe), but nuclei of all the elements have been recognized. To put these kinds of ener- gies in perspective, the previous gen- eration of accelerators for physics ex- Table 4.1. Data on Cosmogenic Nuclides periments, such as the Cornell Elec- -1 tron Storage Ring produce energies in Nuclide Half-life, years Decay constant, yr the 10’s of GeV (1010 eV); while 14C 5730 1.209x 10-4 CERN’s Large Hadron Collider, 3H 12.33 5.62 x 10-2 mankind’s most powerful accelerator, 10Be 1.500 × 106 4.62 x 10-7 located on the Franco-Swiss border 26Al 7.16 × 105 9.68x 10-5 near Geneva produces energies of 36Cl 3.08 × 105 2.25x 10-6 ~10 TeV range (1013 eV). -

Measurement of 10Be from Lake Malawi (Africa) Drill Core Sediments and Implications for Geochronology
Palaeogeography, Palaeoclimatology, Palaeoecology 303 (2011) 110–119 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Measurement of 10Be from Lake Malawi (Africa) drill core sediments and implications for geochronology L.R. McHargue a,⁎, A.J.T. Jull b, A. Cohen a a Department of Geosciences, University of Arizona, 1040 E. Fourth Street, Tucson, Arizona 85721, United States b NSF Arizona AMS Laboratory, University of Arizona, 1118 E. Fourth Street, Tucson, Arizona 85721, United States article info abstract Article history: The cosmogenic radionuclide 10Be was measured from drill core sediments from Lake Malawi in order to Received 19 August 2008 help construct a chronology for the study of the tropical paleoclimate in East Africa. Sediment samples were Received in revised form 29 January 2010 taken every 10 m from the core MAL05-1C to 80 m in depth and then from that depth in core MAL05-1B to Accepted 4 February 2010 382 m. Sediment samples were then later taken at a higher resolution of every 2 m from MAL05-1C. They Available online 12 February 2010 were then leached to remove the authigenic fraction, the leachate was processed to separate out the beryllium isotopes, and 10Be was measured at the TAMS Facility at the University of Arizona. The 10Be/9Be Keywords: fi Beryllium-10 pro le from Lake Malawi sediments is similar to those derived from marine sediment cores for the late Cosmogenic radionuclide Pleistocene, and is consistent with the few radiocarbon and OSL IR measurements made from the same core. Lake sediments Nevertheless, a strong correlation between the stable isotope 9Be and the cosmogenic isotope 10Be suggests Lake Malawi that both isotopes have been well mixed before deposition unlike in some marine sediment cores. -

Ocean Drilling Program Scientific Results Volume
9. GEOCHEMICAL EXPRESSION OF EARLY DIAGENESIS IN MIDDLE EOCENE-LOWER OLIGOCENE PELAGIC SEDIMENTS IN THE SOUTHERN LABRADOR SEA, SITE 647, ODP LEG 1051 M. A. Arthur,2 W. E. Dean,3 J. C. Zachos,2 M. Kaminski,4 S. Hagerty Rieg,2 and K. Elmstrom2 ABSTRACT Geochemical analyses of the middle Eocene through lower Oligocene lithologic Unit IIIC (260-518 meters below seafloor [mbsf]) indicate a relatively constant geochemical composition of the detrital fraction throughout this deposi tional interval at Ocean Drilling Program (ODP) Site 647 in the southern Labrador Sea. The main variability occurs in redox-sensitive elements (e.g., iron, manganese, and phosphorus), which may be related to early diagenetic mobility in anaerobic pore waters during bacterial decomposition of organic matter. Initial preservation of organic matter was me diated by high sedimentation rates (36 m/m.y.). High iron (Fe) and manganese (Mn) contents are associated with car bonate concretions of siderite, manganosiderite, and rhodochrosite. These concretions probably formed in response to elevated pore-water alkalinity and total dissolved carbon dioxide (C02) concentrations resulting from bacterial sulfate reduction, as indicated by nodule stable-isotope compositions and pore-water geochemistry. These nodules differ from those found in upper Cenozoic hemipelagic sequences in that they are not associated with methanogenesis. Phosphate minerals (carbonate-fluorapatite) precipitated in some intervals, probably as the result of desorption of phosphorus from iron and manganese during reduction. The bulk chemical composition of the sediments differs little from that of North Atlantic Quaternary abyssal red clays, but may contain a minor hydrothermal component. The silicon/ aluminum (Si/Al) ratio, however, is high and variable and probably reflects original variations in biogenic opal, much of which is now altered to smectite and/or opal CT. -

12 Natural Isotopes of Elements Other Than H, C, O
12 NATURAL ISOTOPES OF ELEMENTS OTHER THAN H, C, O In this chapter we are dealing with the less common applications of natural isotopes. Our discussions will be restricted to their origin and isotopic abundances and the main characteristics. Only brief indications are given about possible applications. More details are presented in the other volumes of this series. A few isotopes are mentioned only briefly, as they are of little relevance to water studies. Based on their half-life, the isotopes concerned can be subdivided: 1) stable isotopes of some elements (He, Li, B, N, S, Cl), of which the abundance variations point to certain geochemical and hydrogeological processes, and which can be applied as tracers in the hydrological systems, 2) radioactive isotopes with half-lives exceeding the age of the universe (232Th, 235U, 238U), 3) radioactive isotopes with shorter half-lives, mainly daughter nuclides of the previous catagory of isotopes, 4) radioactive isotopes with shorter half-lives that are of cosmogenic origin, i.e. that are being produced in the atmosphere by interactions of cosmic radiation particles with atmospheric molecules (7Be, 10Be, 26Al, 32Si, 36Cl, 36Ar, 39Ar, 81Kr, 85Kr, 129I) (Lal and Peters, 1967). The isotopes can also be distinguished by their chemical characteristics: 1) the isotopes of noble gases (He, Ar, Kr) play an important role, because of their solubility in water and because of their chemically inert and thus conservative character. Table 12.1 gives the solubility values in water (data from Benson and Krause, 1976); the table also contains the atmospheric concentrations (Andrews, 1992: error in his Eq.4, where Ti/(T1) should read (Ti/T)1); 2) another category consists of the isotopes of elements that are only slightly soluble and have very low concentrations in water under moderate conditions (Be, Al). -

4. a Growth Model for Polymetallic Nodules
A GEOLOGICAL MODEL OF POLYMETALLIC NODULE DEPOSITS IN THE CLARION‐CLIPPERTON FRACTURE ZONE ISA TECHNICAL STUDY SERIES Technical Study No. 1 Global Non‐Living Resources on the Extended Continental Shelf: Prospects at the year 2000 Technical Study No. 2 Polymetallic Massive Sulphides and Cobalt‐Rich Ferromanganese Crusts: Status and Prospects Technical Study No. 3 Biodiversity, Species Ranges and Gene Flow in the Abyssal Pacific Nodule Province: Predicting and Managing the Impacts of Deep Seabed Mining Technical Study No. 4 Issues associated with the Implementation of Article 82 of the United Nations Convention on the Law of the Sea Technical Study No. 5 Non‐Living resources of the Continental Shelf beyond 200 nautical miles: Speculations on the Implementation of Article 82 of the United Nations Convention on the Law of the Sea PAGE | II A GEOLOGICAL MODEL OF POLYMETALLIC NODULE DEPOSITS IN THE CLARION‐ CLIPPERTON FRACTURE ZONE This report contains a summary of two documents – A Geological Model of Polymetallic Nodule Deposits in the Clarion‐Clipperton Fracture Zone and a Prospector’s Guide prepared under the project ‘Development of a Geological Model of Polymetallic Nodule Deposits in the Clarion‐Clipperton Fracture Zone, Pacific Ocean’. ISA TECHNICAL STUDY: NO. 6 International Seabed Authority Kingston, Jamaica PAGE | III The designation employed and the presentation of materials in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the International Seabed Authority concerning the legal status of any country or territory or of its authorities, or concerning the delimitation of its frontiers or maritime boundaries. All rights reserved. -

Beryllium-10 Terrestrial Cosmogenic Nuclide Surface Exposure Dating of Quaternary Landforms in Death Valley
Geomorphology 125 (2011) 541–557 Contents lists available at ScienceDirect Geomorphology journal homepage: www.elsevier.com/locate/geomorph Beryllium-10 terrestrial cosmogenic nuclide surface exposure dating of Quaternary landforms in Death Valley Lewis A. Owen a,⁎, Kurt L. Frankel b, Jeffrey R. Knott c, Scott Reynhout a, Robert C. Finkel d,e, James F. Dolan f, Jeffrey Lee g a Department of Geology, University of Cincinnati, Cincinnati, Ohio, USA b School of Earth and Atmospheric Sciences, Georgia Institute of Technology, Atlanta, GA 30332, USA c Department of Geological Sciences, California State University, Fullerton, California, USA d Department of Earth and Planetary Science Department, University of California, Berkeley, Berkeley, CA 94720-4767, USA e CEREGE, BP 80 Europole Méditerranéen de l'Arbois, 13545 Aix en Provence Cedex 4, France f Department of Earth Sciences, University of Southern California, Los Angeles, California, USA g Department of Geological Sciences, Central Washington University, Ellensburg, Washington, USA article info abstract Article history: Quaternary alluvial fans, and shorelines, spits and beach bars were dated using 10Be terrestrial cosmogenic Received 10 March 2010 nuclide (TCN) surface exposure methods in Death Valley. The 10Be TCN ages show considerable variance on Received in revised form 3 October 2010 individual surfaces. Samples collected in the active channels date from ~6 ka to ~93 ka, showing that there is Accepted 18 October 2010 significant 10Be TCN inheritance within cobbles and boulders. This -
(12) Patent Application Publication (10) Pub. No.: US 2005/0254988A1 Wagner (43) Pub
US 2005O254988A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0254988A1 Wagner (43) Pub. Date: Nov. 17, 2005 (54) METALALLOY AND METALALLOY Publication Classification STORAGE PRODUCT FOR STORING EAST NEUTRON EMITTERS (51) Int. Cl. ................................................. C22C 43/00 (52) U.S. Cl. .................................................................. 420/1 (75) Inventor: Anthony S. Wagner, Lakeway, TX (US) (57) ABSTRACT Correspondence Address: THE CULBERTSON GROUP, PC. 1114 LOST CREEK BLVD. Aliquid reactant metal alloy includes at least one chemically SUTE 420 active metal for reacting with non-radioactive material in a AUSTIN, TX 78746 (US) mixed waste Stream being treated. The reactant alloy also includes at least one radiation absorbing metal. Radioactive (73) Assignee: Clean Technologies International Cor isotopes in the waste Stream, including any fast neutron poration emitting isotopes alloy with, or disperse in, the chemically Appl. No.: 11/173,271 active metal and the radiation absorbing metals are able to (21) absorb a significant portion of the radioactive emissions (22) Filed: Jul. 1, 2005 asSociated with the isotopes. A transmutation target fraction is included for absorbing fast neutrons and a transmutation Related U.S. Application Data emission absorbing fraction is provided for absorbing emis sions that result from the absorption of a fast neutron by the (63) Continuation of application No. 10/059,808, filed on transmutation target fraction. Non-radioactive constituents Jan. 29, 2002, which is a continuation-in-part of in the waste material are broken down into harmless and application No. 09/334,985, filed on Jun. 17, 1999, useful constituents, leaving the alloyed radioactive isotopes now Pat. -

Polar Desert Chronologies Through Quantitative Measurements of Salt Accumulation
Polar desert chronologies through quantitative measurements of salt accumulation Joseph A. Graly1, Kathy J. Licht1, Gregory K. Druschel1, and Michael R. Kaplan2 1Department of Earth Sciences, Indiana University–Purdue University Indianapolis, Indianapolis, Indiana 46202, USA 2Lamont–Doherty Earth Observatory, Palisades, New York 10964, USA ABSTRACT isotopes suggest a primarily atmospheric ori- We measured salt concentration and speciation in the top horizons of moraine sediments gin, with some trace mineral boron (Leslie et from the Transantarctic Mountains (Antarctica) and compared the salt data to cosmogenic- al., 2014). However, the Dry Valleys boron iso- nuclide exposure ages on the same moraine. Because the salts are primarily of atmospheric tope values that suggest a trace mineral compo- origin, and their delivery to the sediment is constant over relevant time scales, a linear rate of nent could also form from Rayleigh distillation accumulation is expected. When salts are measured in a consistent grain-size fraction and at during the precipitation of atmospheric boron a consistent position within the soil column, a linear correlation between salt concentration (Rose-Koga et al., 2006). and exposure age is evident. This correlation is strongest for boron-containing salts (R2 > 0.99), Atmospheric aerosols exist either as acid 2 but is also strong (R ≈ 0.9) for most other water-extracted salt species. The relative mobility vapors and gases (e.g., HNO3, N2O5, etc.) or of salts in the soil column does not correspond to species solubility (borate is highly soluble). as anion-cation pairs, commonly adhering to Instead, the highly consistent behavior of boron within the soil column is best explained by particulate matter. -

ECONOMIC ASPECTS of NODULE MINING Although Deep-Sea
CHAPTER 11 ECONOMIC ASPECTS OF NODULE MINING J. L. MERO INTRODUCTION Although deep-sea manganese nodules were discovered over a'century ago by scientists of the HMS Challenger expedition, few analyses of the nodules for the economically significant elements such as nickel, copper, cobalt and molybdenum were made in those early days and no consideration was given to these deposits as a possible commercial source of metals until the early 1950's when the mining of the nodules was advocated as a possible source of manganese (Mero, 1952). As the result of a haul of nodules taken in relatively shallow water (900 m) about 370 km east of Tahiti on the western edge of the Tuamoto Plateau during the 1957-58 International Geophysical Year (the nodules contained about 2% of cobalt, a valuable metal at that particular time), a study was initiated by the Institute of Marine Resources of the University of California to determine if it might be economic to mine and process the nodules for their cobalt, nickel and copper contents. The results of that study were favourable' with respect to the technical and economic factors involved in mining and processing the nodules. All the research and development in this matter dates from the release of the report describing the results of that study (Mero, 1958). To the present time (1975) over $150 million* has been expended in the exploration of the nodule deposits and in the development of mining and processing systems. An additional $100 million is to be spent in the next few years in these activities. -

Study of the Structure Model of Todorokite
Title Study of the Structure Model of Todorokite Author(s) Miura, Hiroyuki Citation 北海道大学理学部紀要, 23(1), 41-51 Issue Date 1991-07 Doc URL http://hdl.handle.net/2115/36772 Type bulletin (article) File Information 23-1_p41-51.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Jour. Fac. Sci., Hokkaido Univ., Ser. IV, vol. 23, no. 1, July., 1991, pp. 41 -51 STUDY OF THE STRUCTURE MODEL OF TODOROKITE by Hiroyuki Miura (with 5 text-figures and 3 tables) Abstract The X - ray diffraction study of todorokite from Japan was carried out and appropriate struc tural model was calculated by Rietveld method. The calculation of the theoretical diffraction pattern was done for a layer structural model and the calculated results show that the layer model can explain the observed diffraction data. Some of the properties of todorokite such as superstructure or shrinkage of c-axis on heating can be explained by the layer model which con tains random H 20 between the [MnOs] layers. Introduction Todorokite is a manganese oxide mineral having H 20 in its structure. It was reported first from the Todoroki gold mine, Hokkaido, Japan (Yoshimura, 1934) . This mineral occurs as aggregate of fine acicular crystals. It also contains H20, Ca, Ba and Mg. The X -ray powder diffraction data of todorokite clearly show diffraction peaks of 9.6, 4.8, 3.2, 2.45 and 1.42 A. Since 1934 there has been many reports of todorokite like minerals from all over the world (Frondel, 1953; Frondel et aI. , 1960; Ljunggren, 1960; Straczek et aI., 1960; Faulring, 1961 ; Larson, 1962; Radtke et aI., 1967; Lawrence et aI., 1968; Harada, 1982; Siegel and Turner, 1983) .